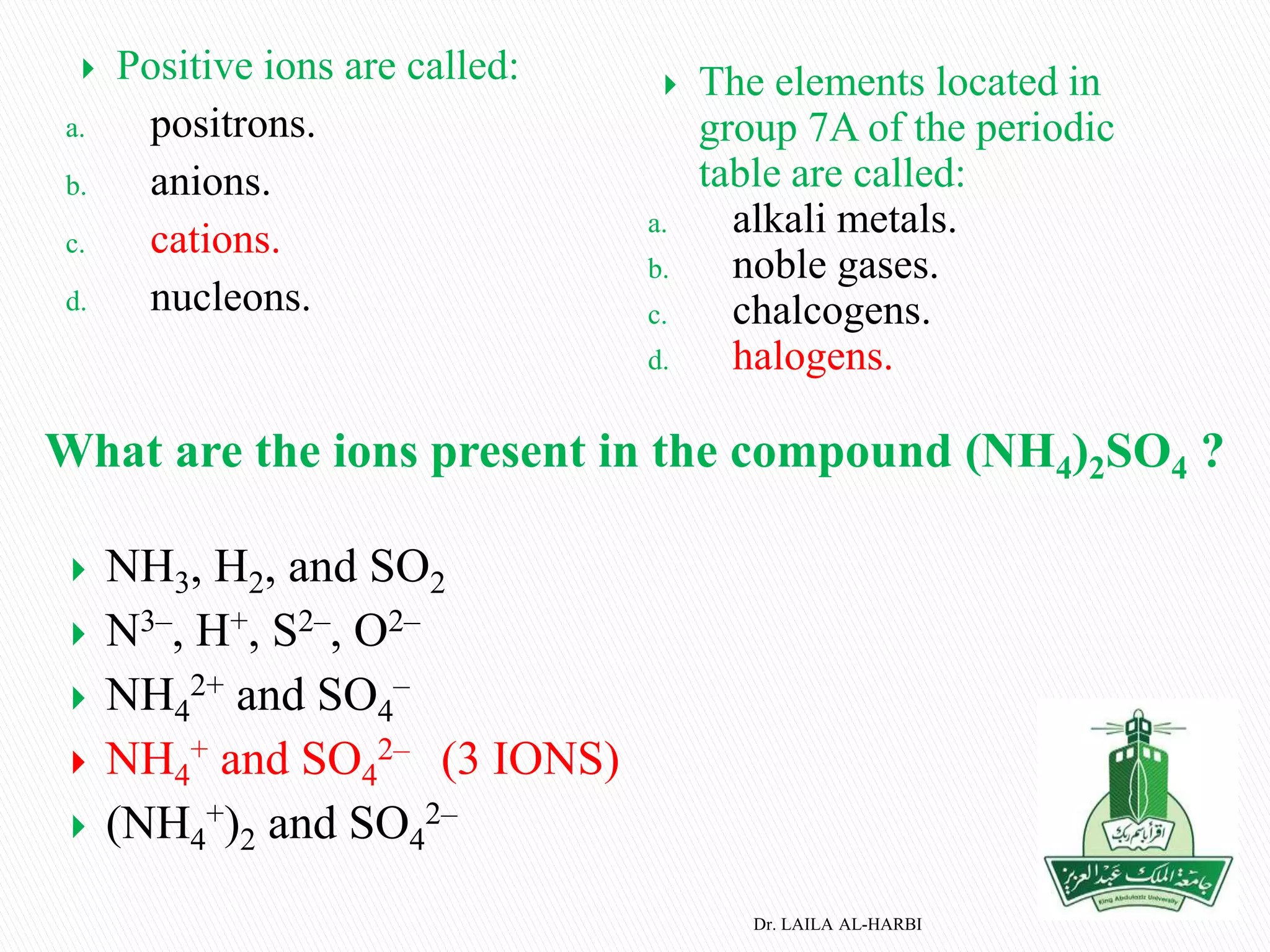
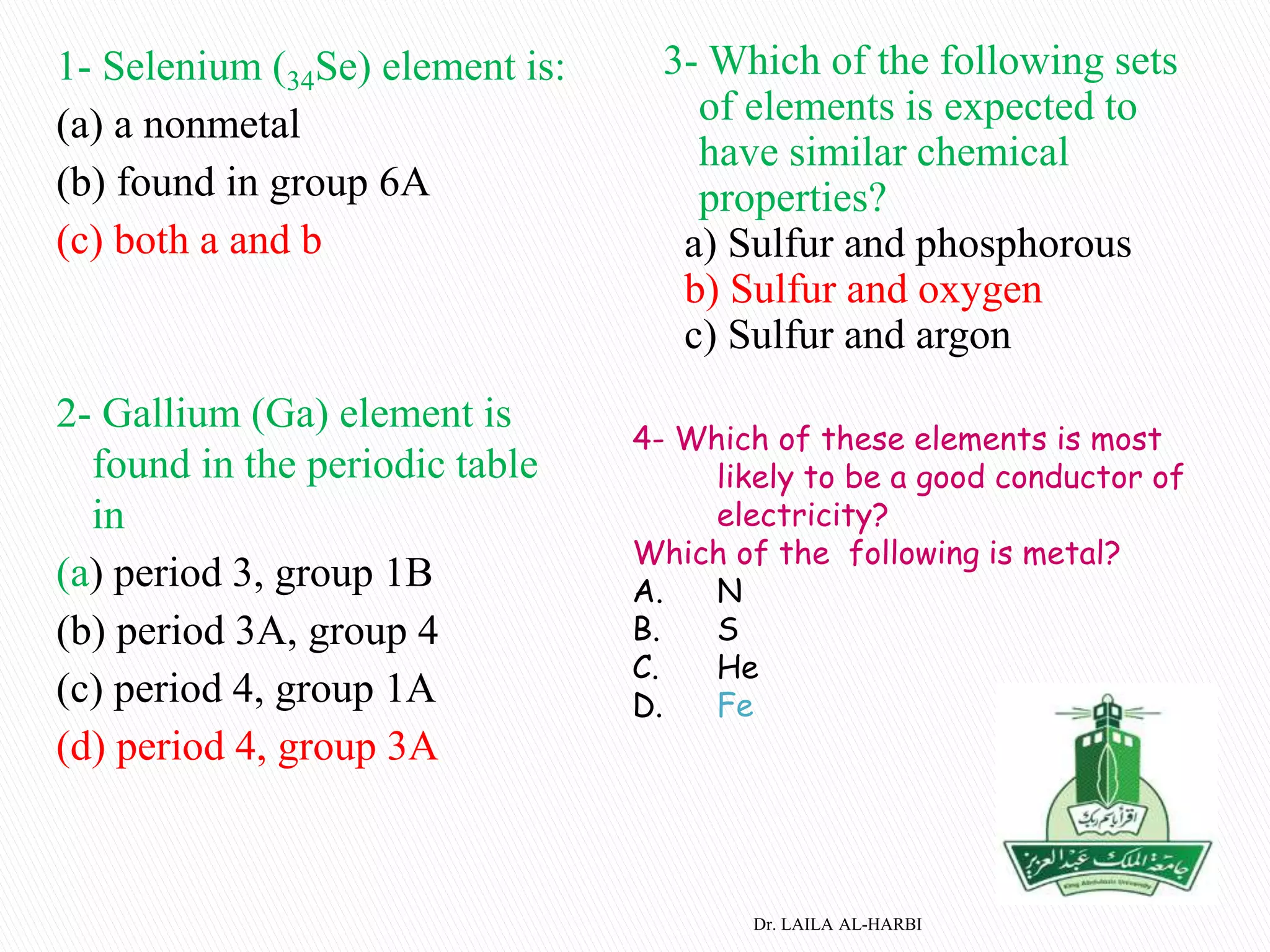
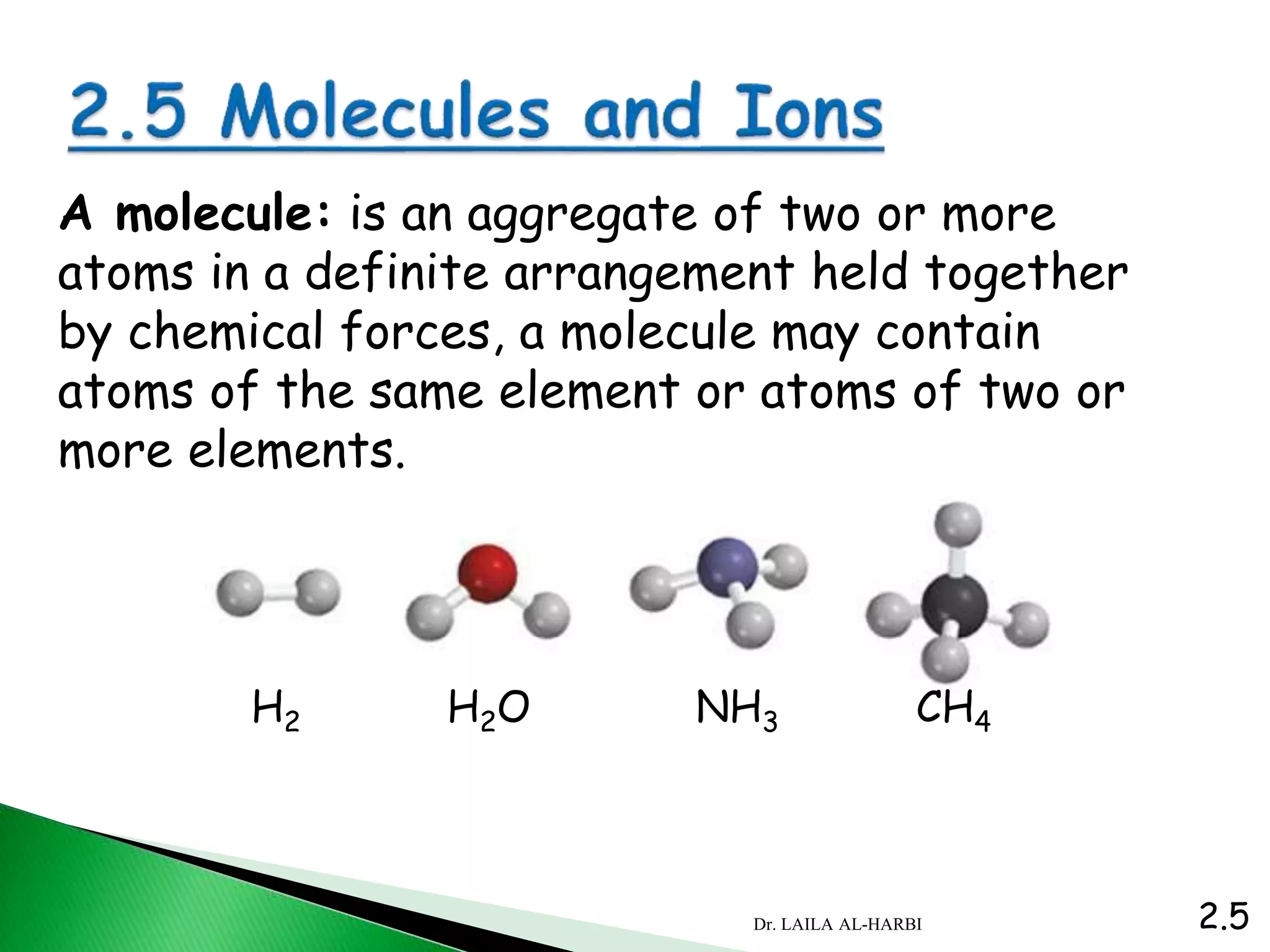
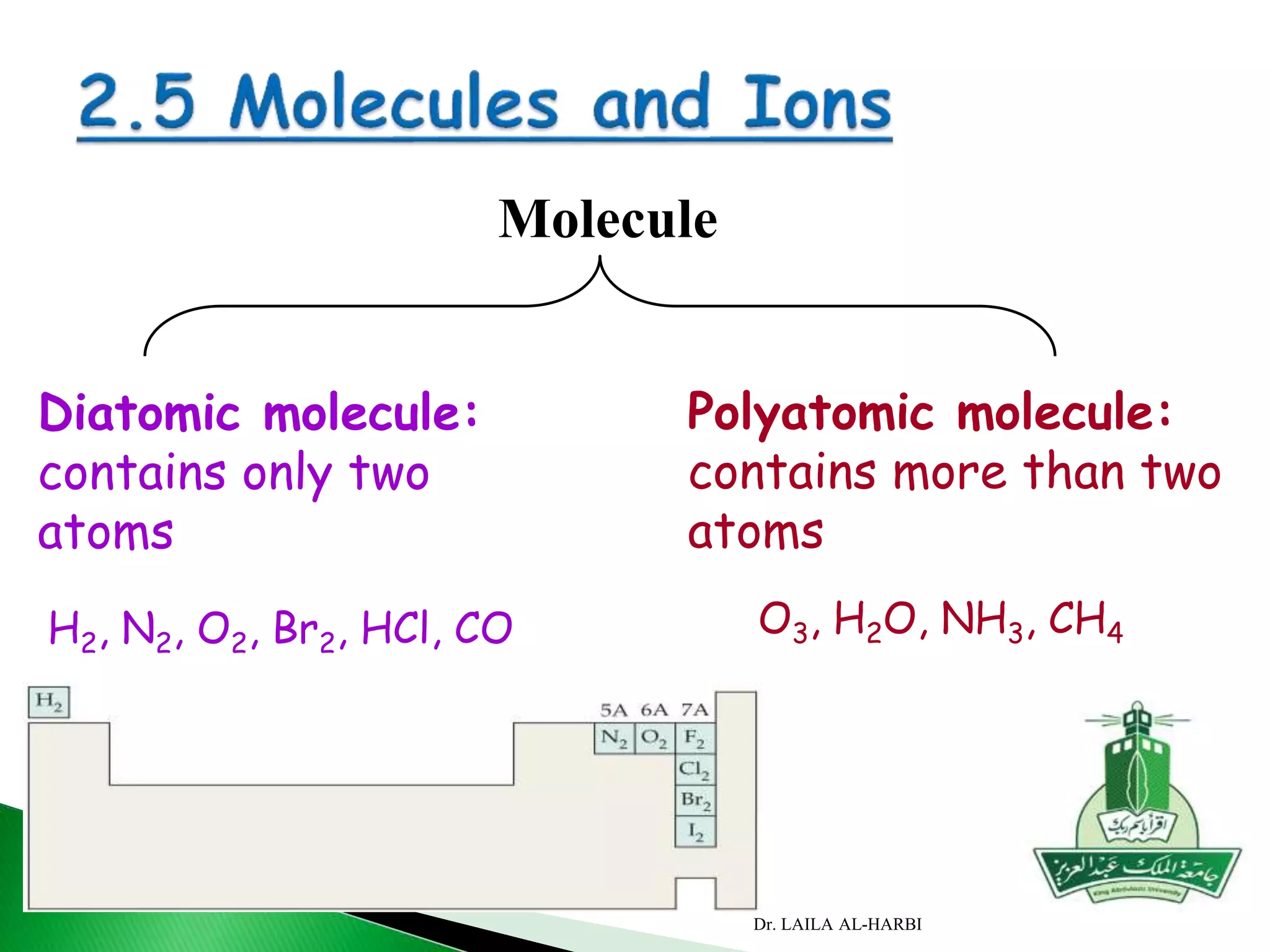
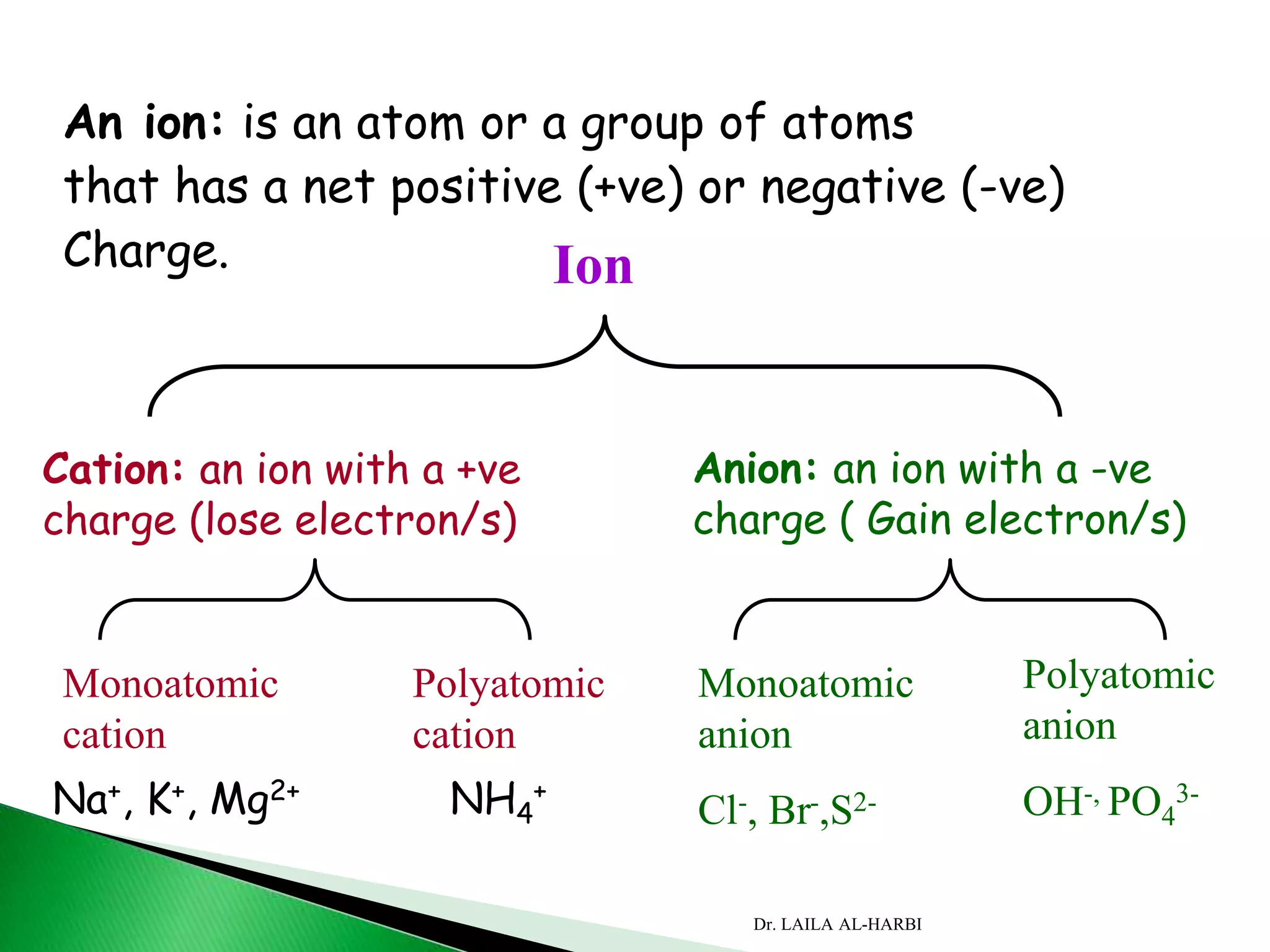
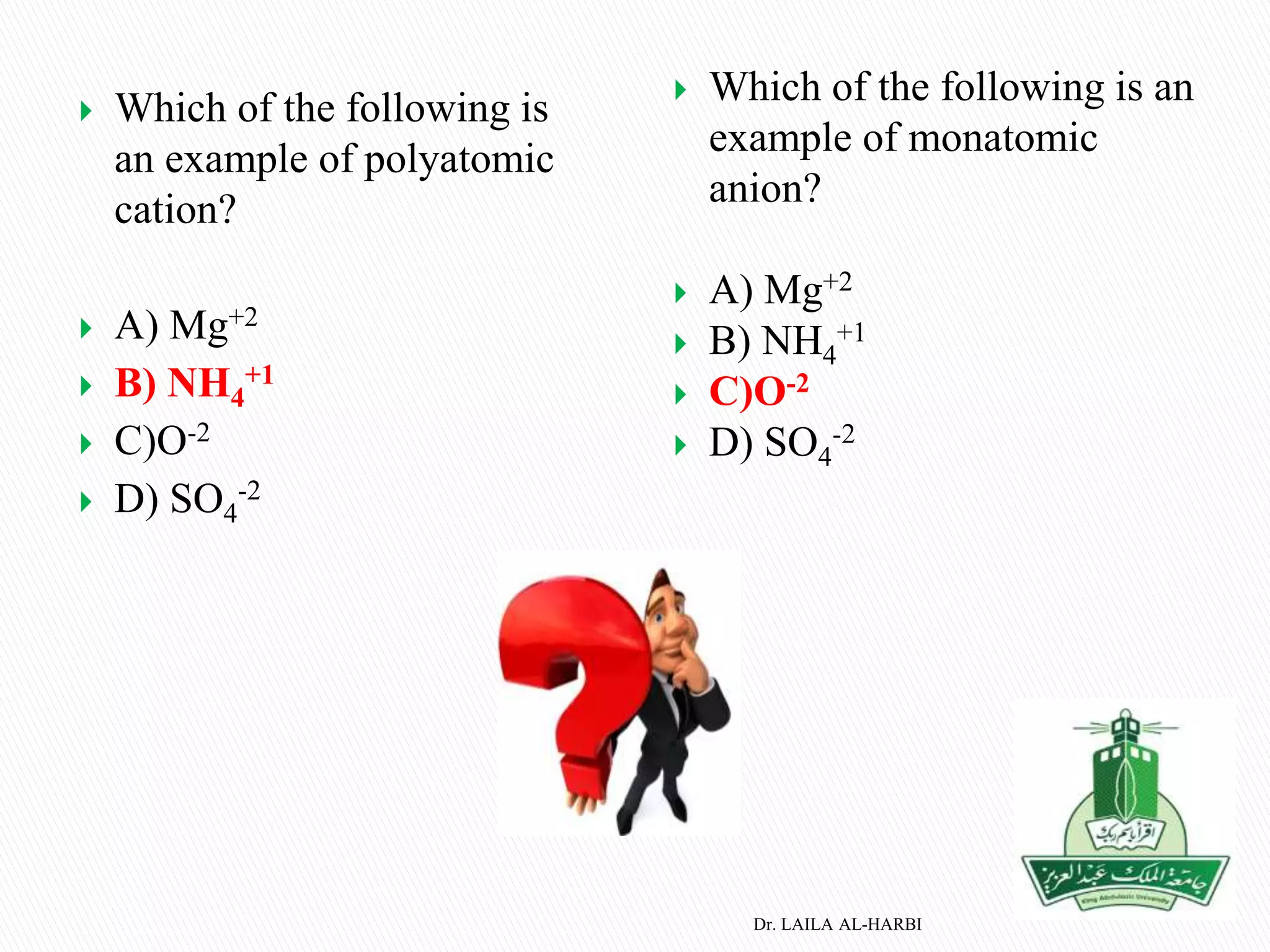
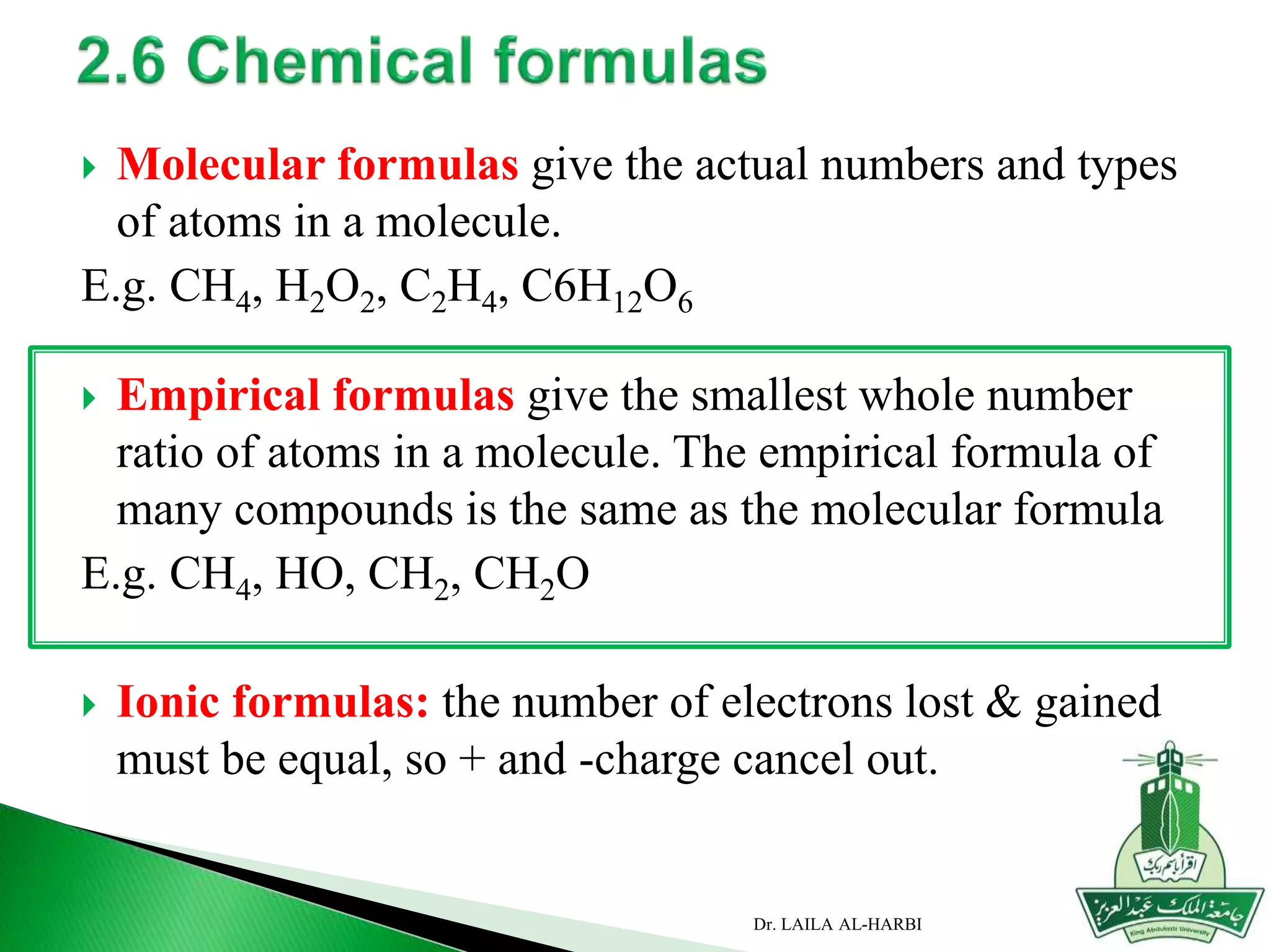
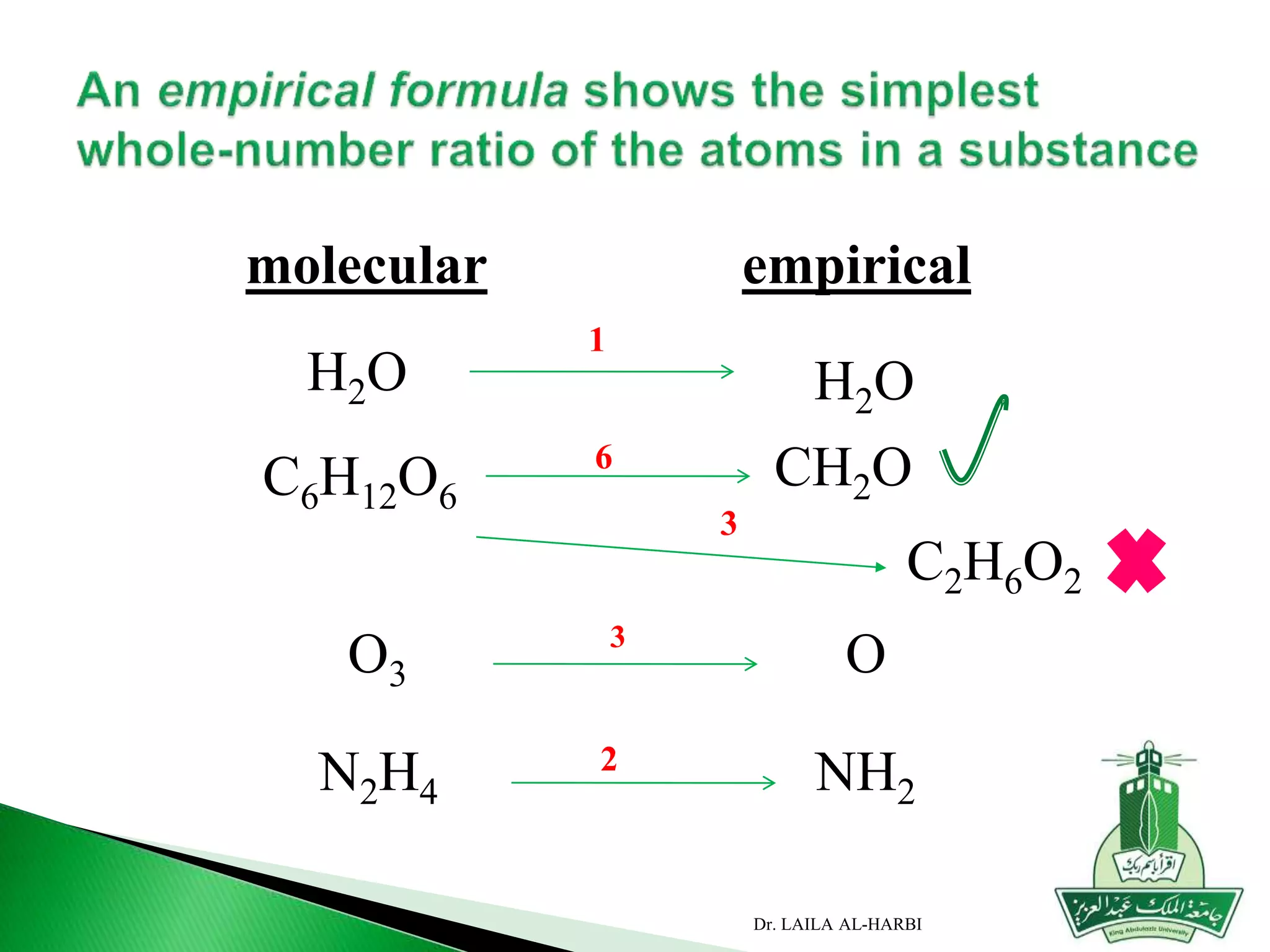
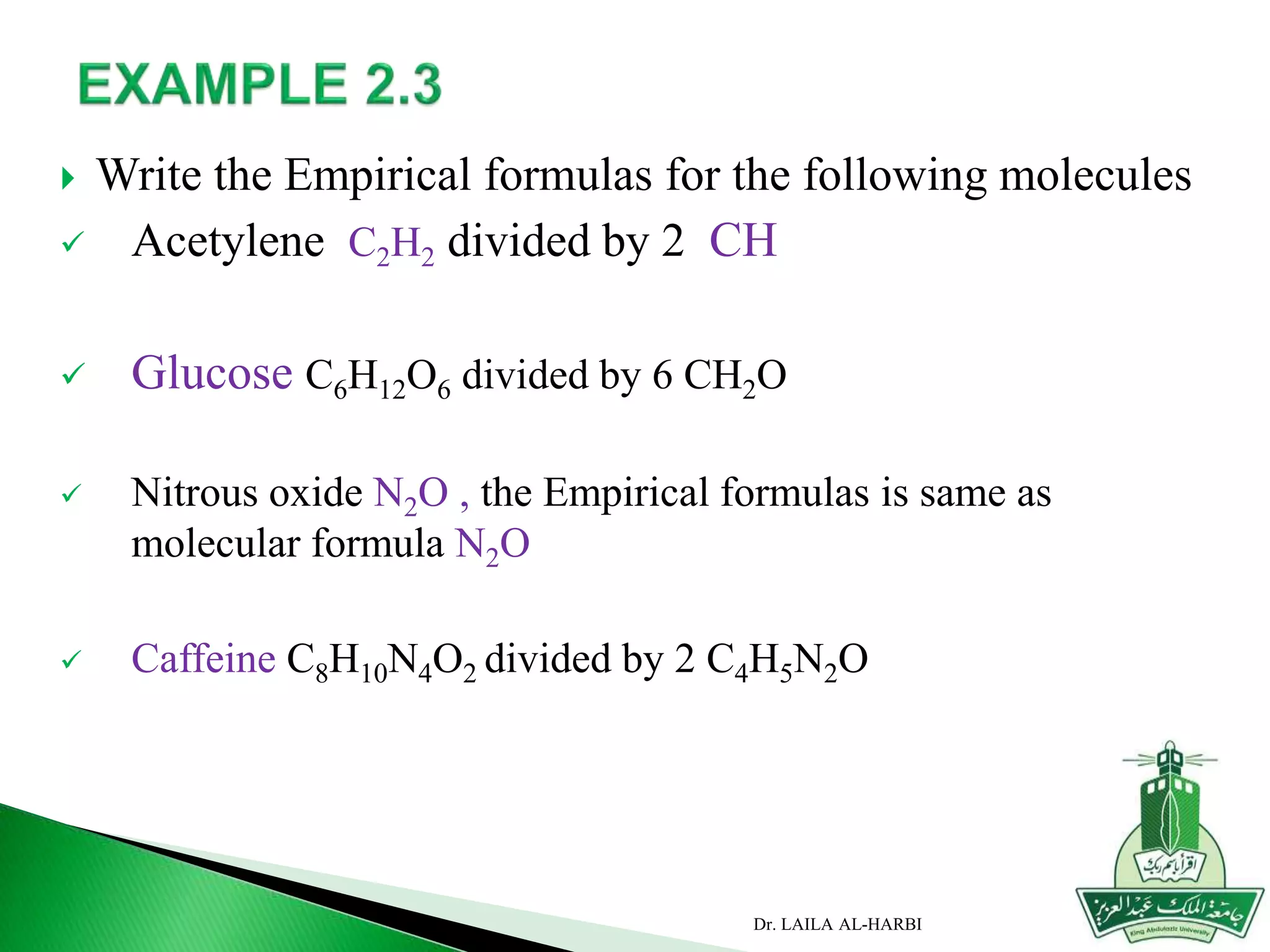
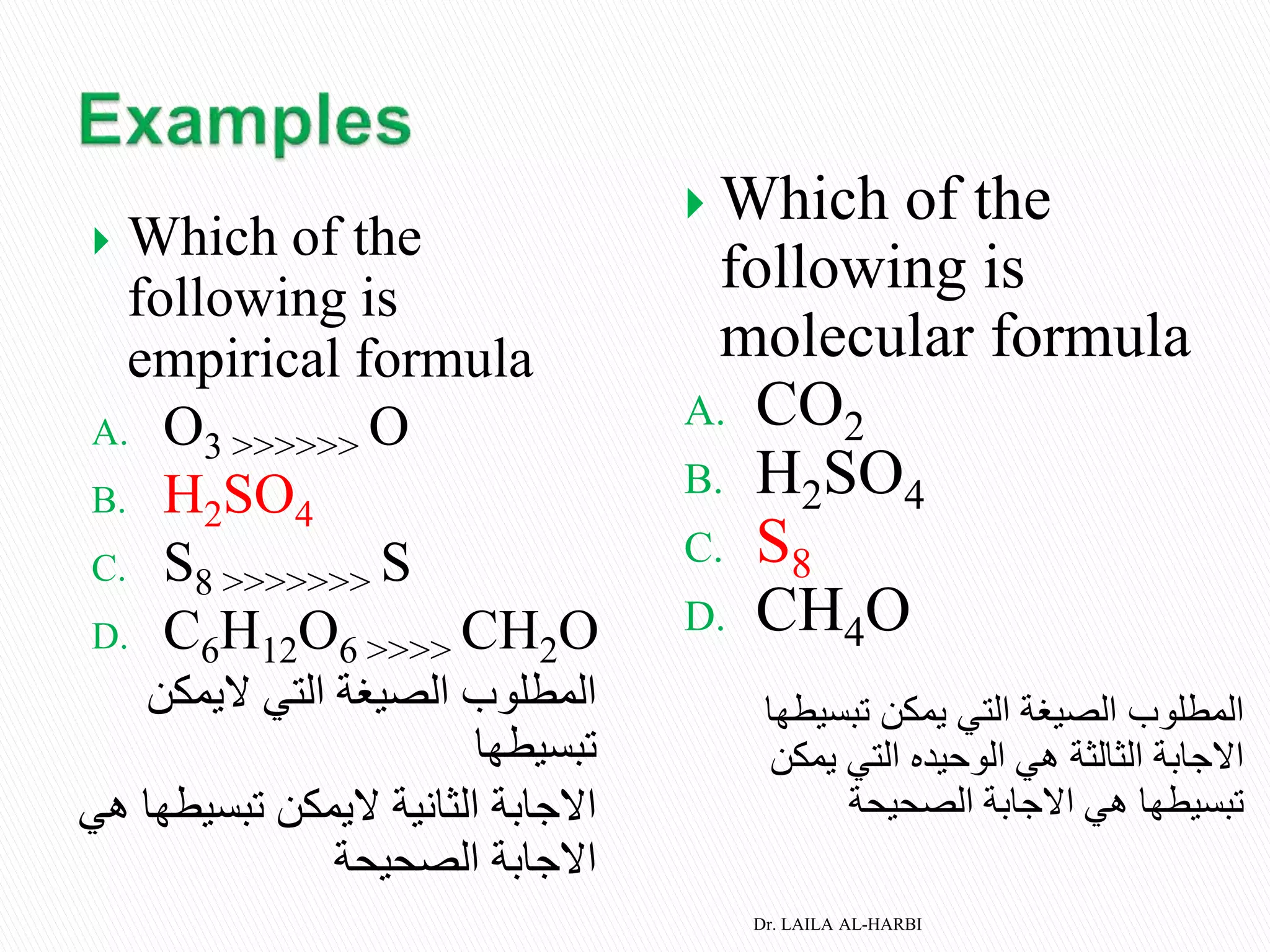
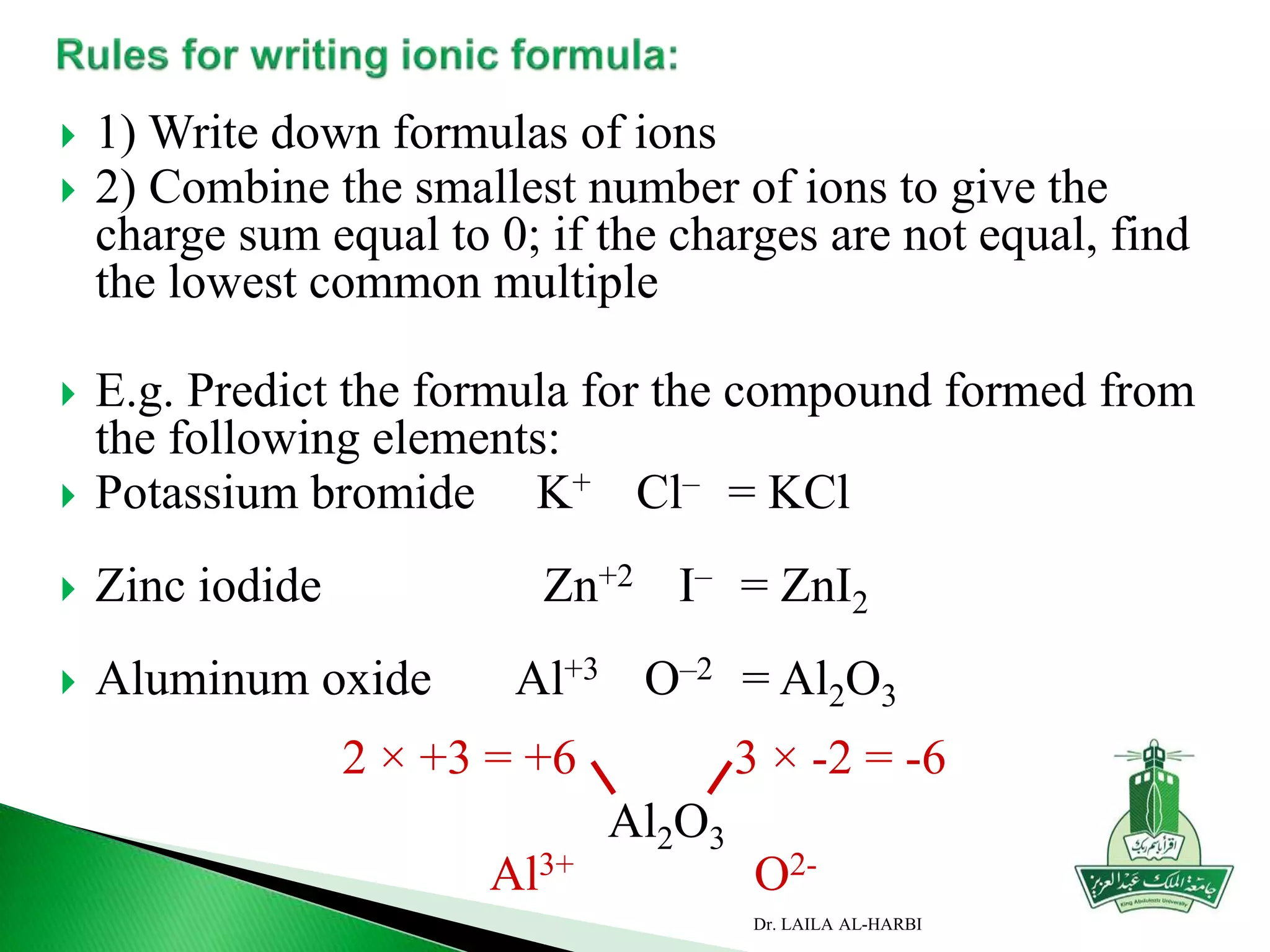
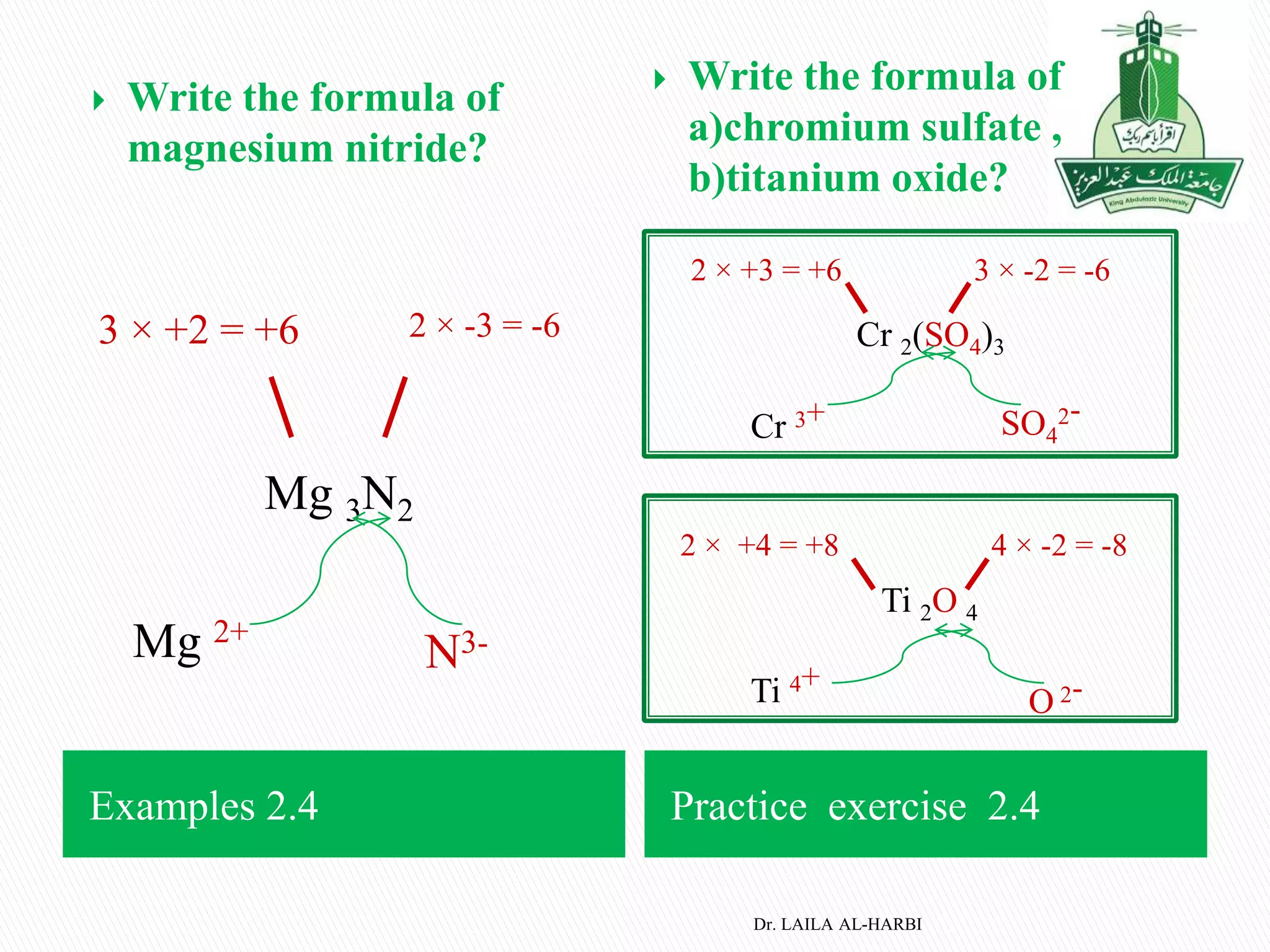
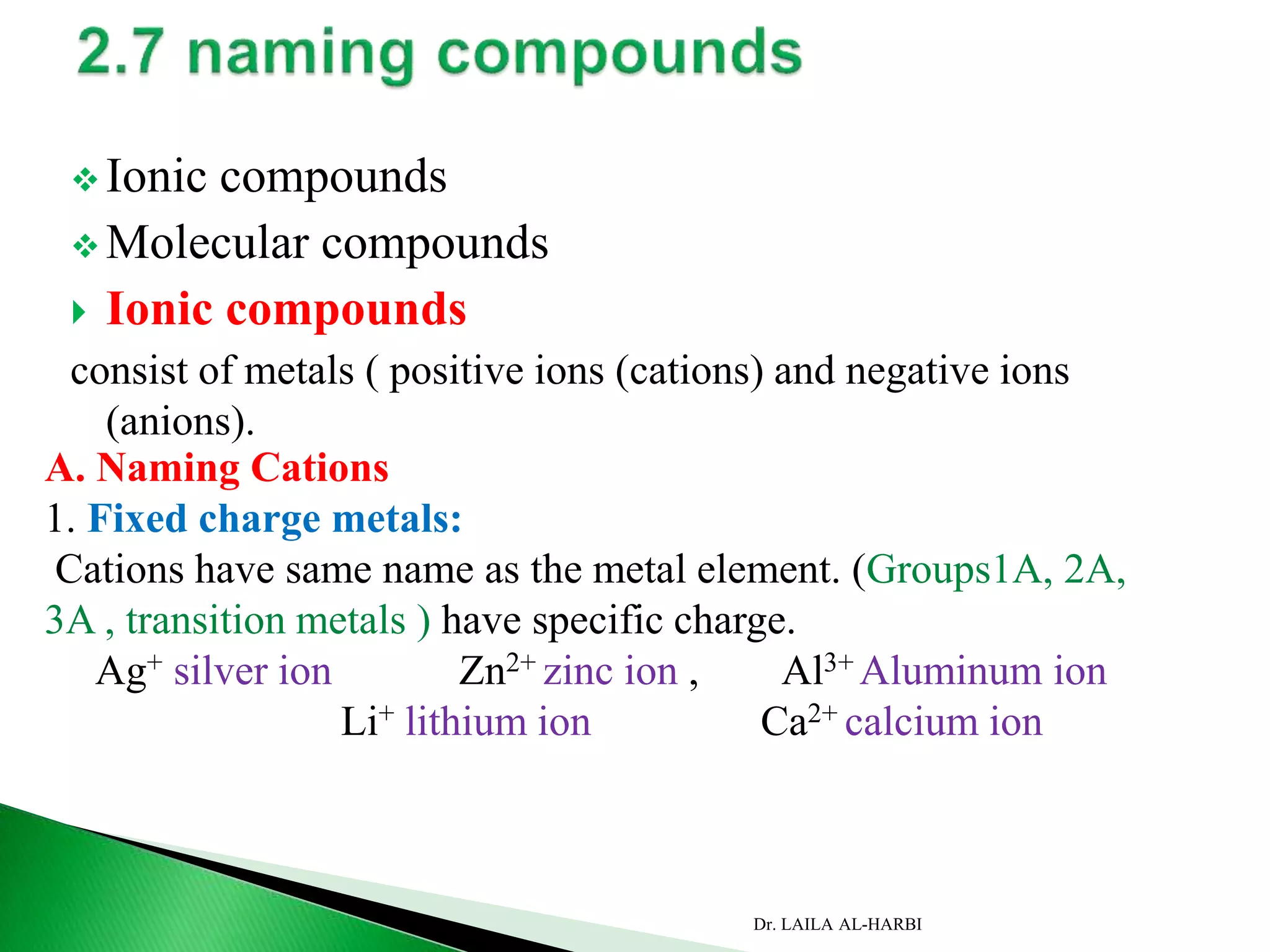
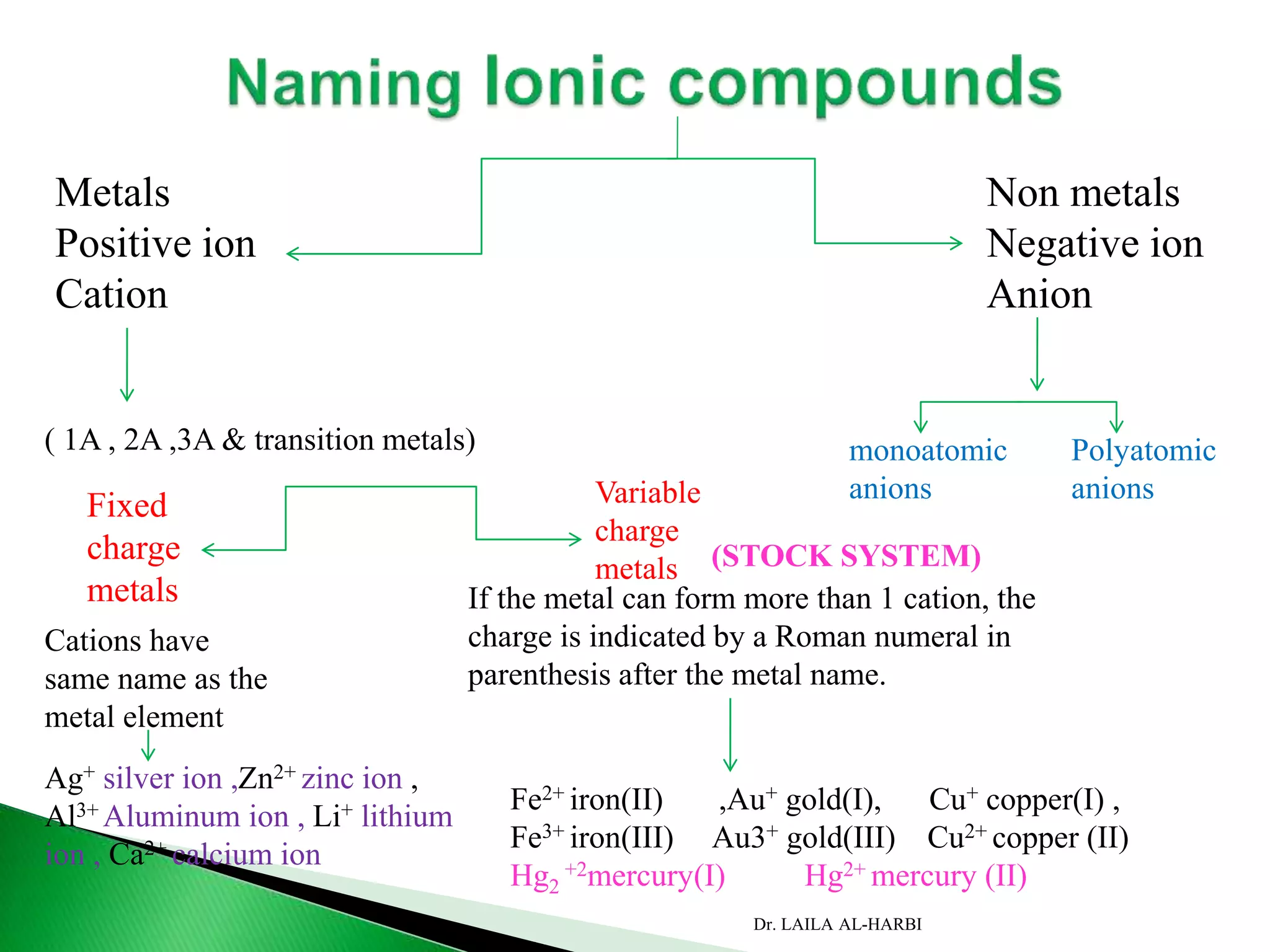
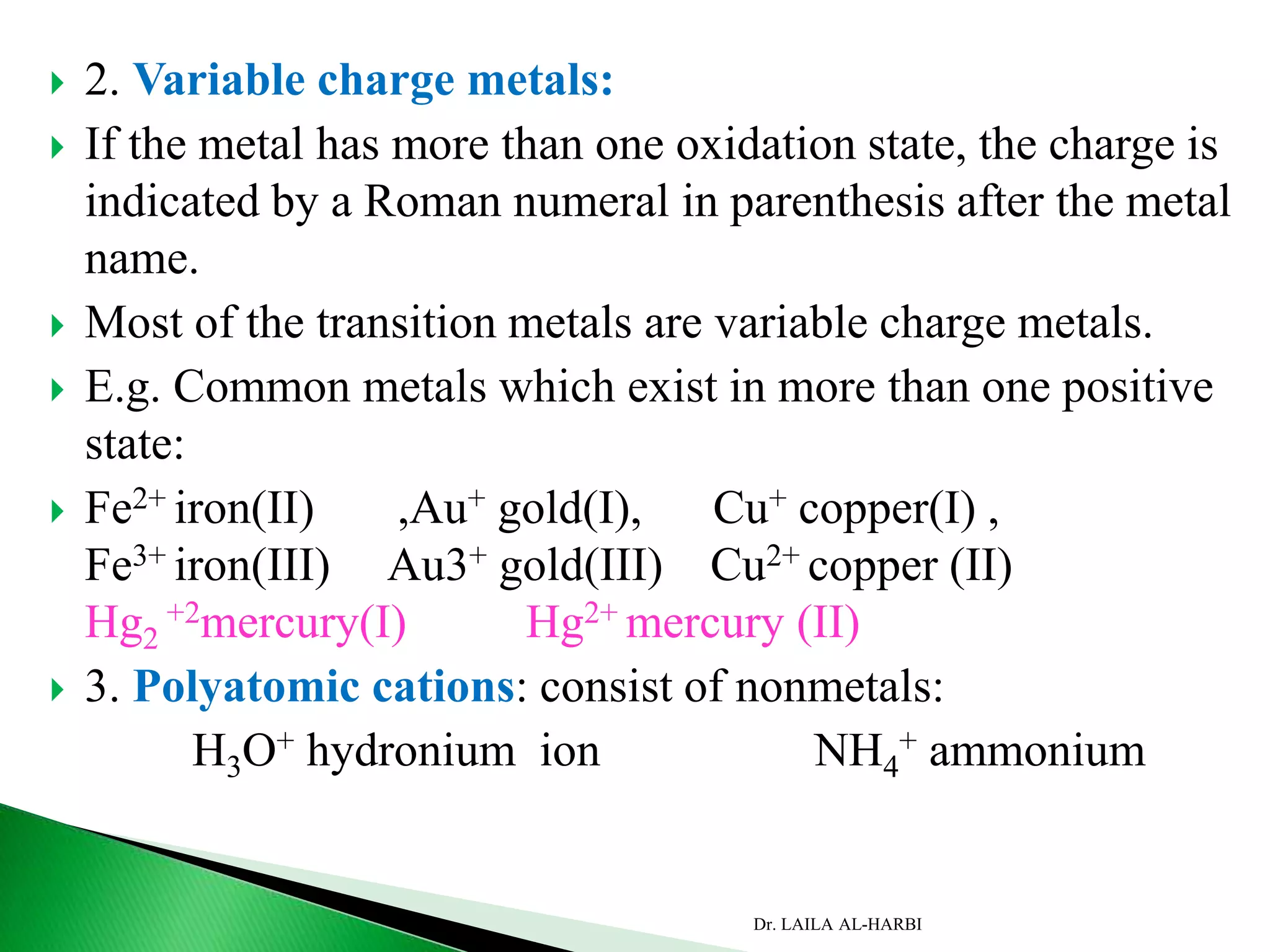
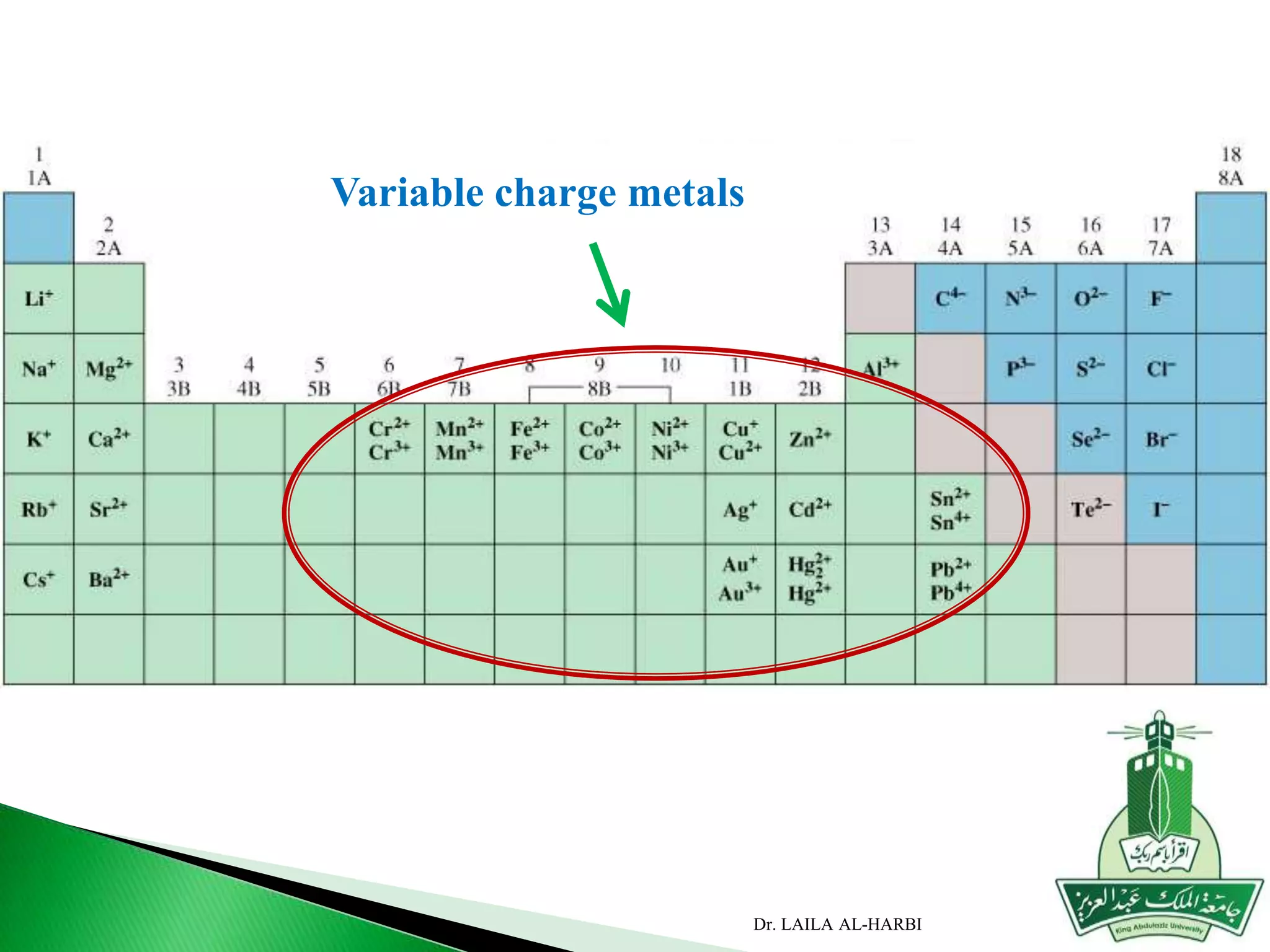
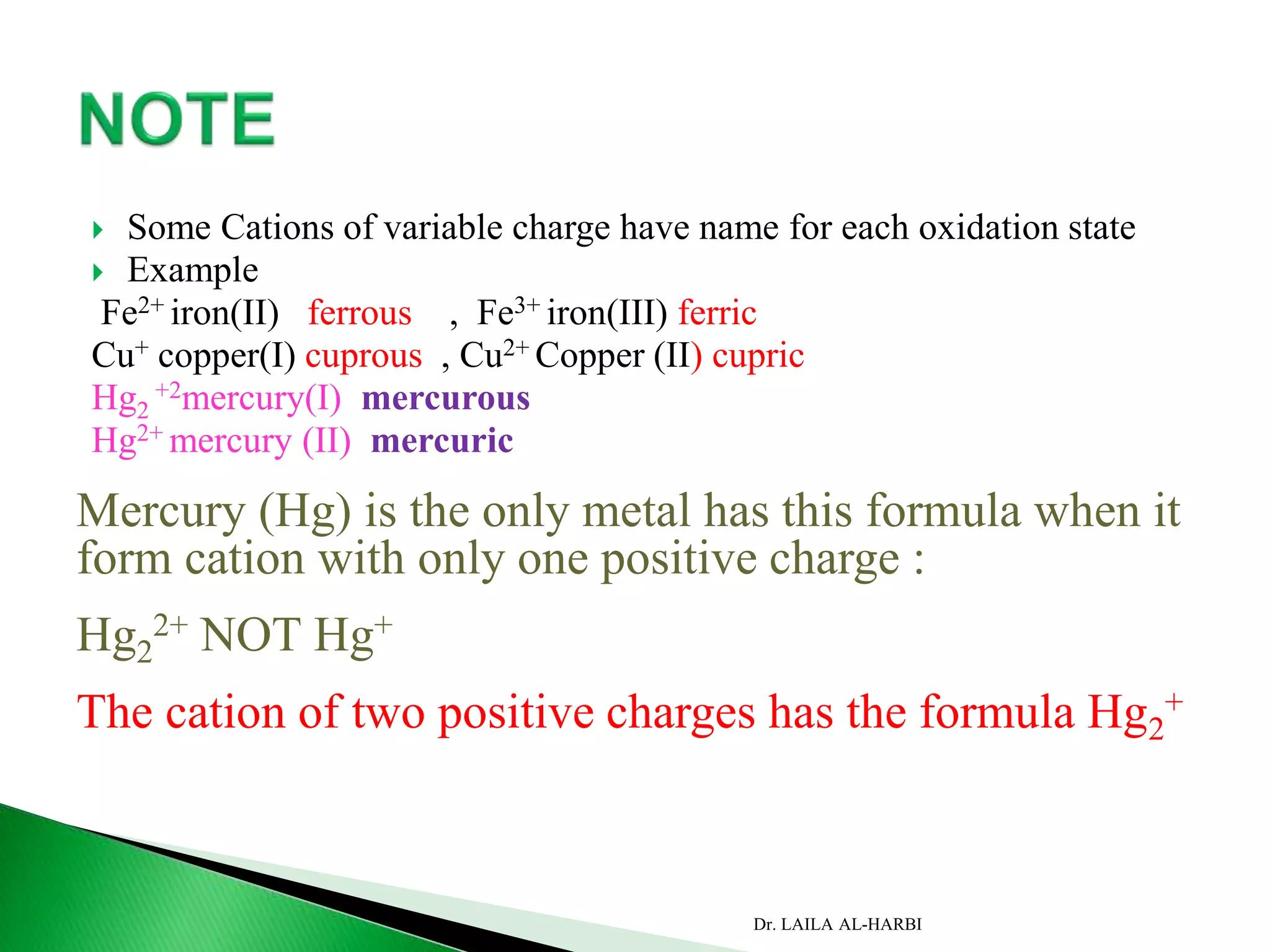
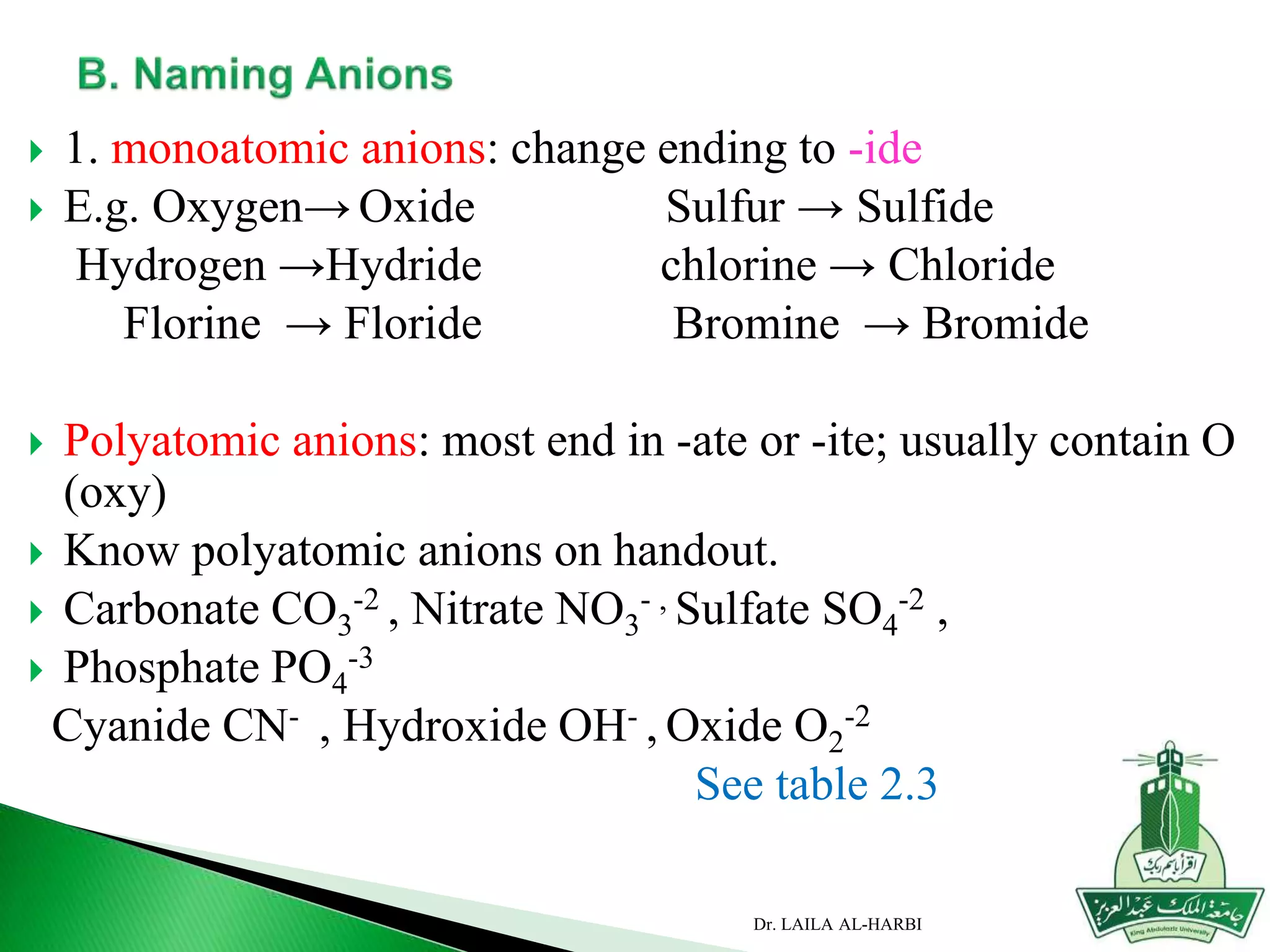
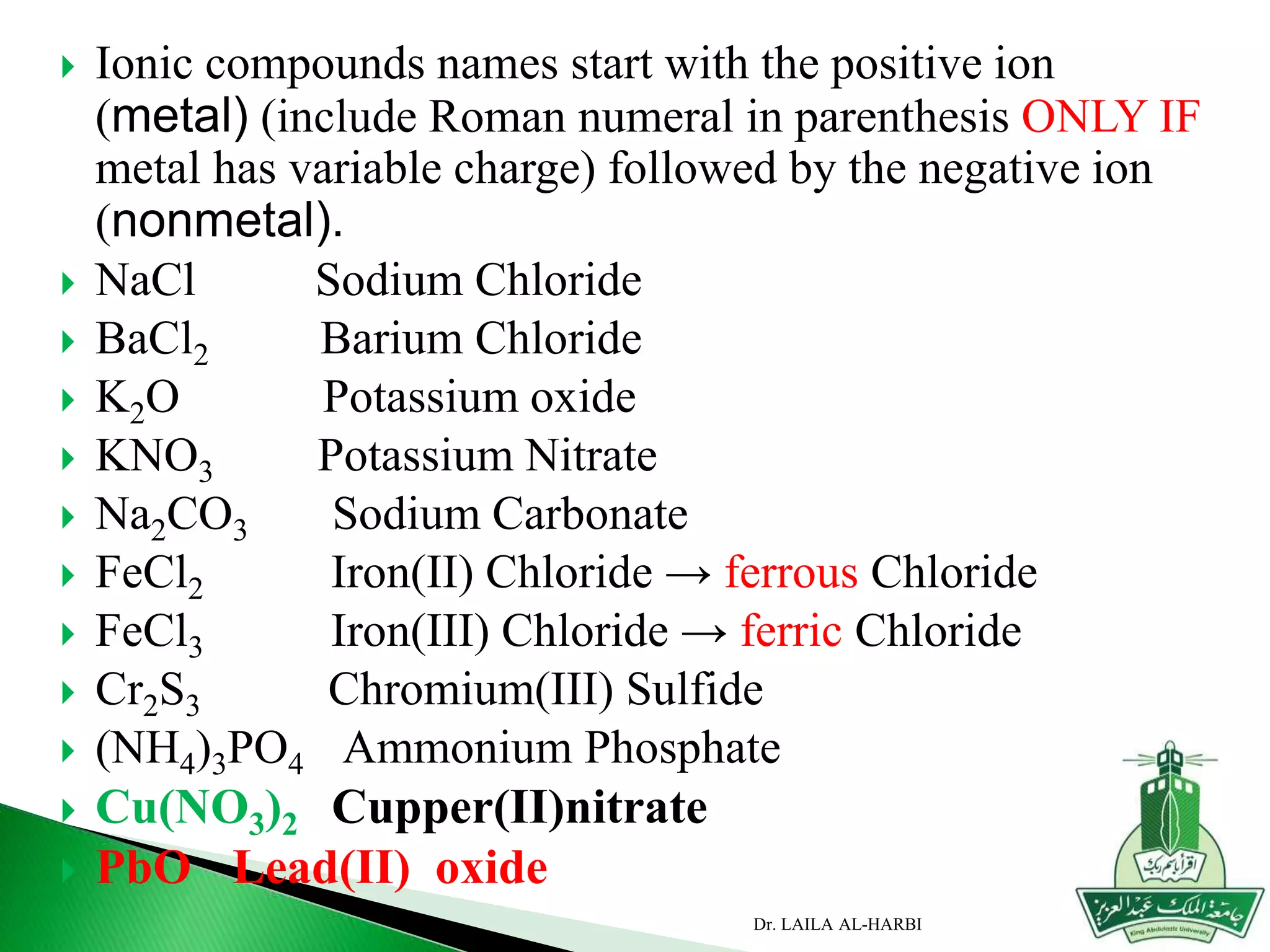
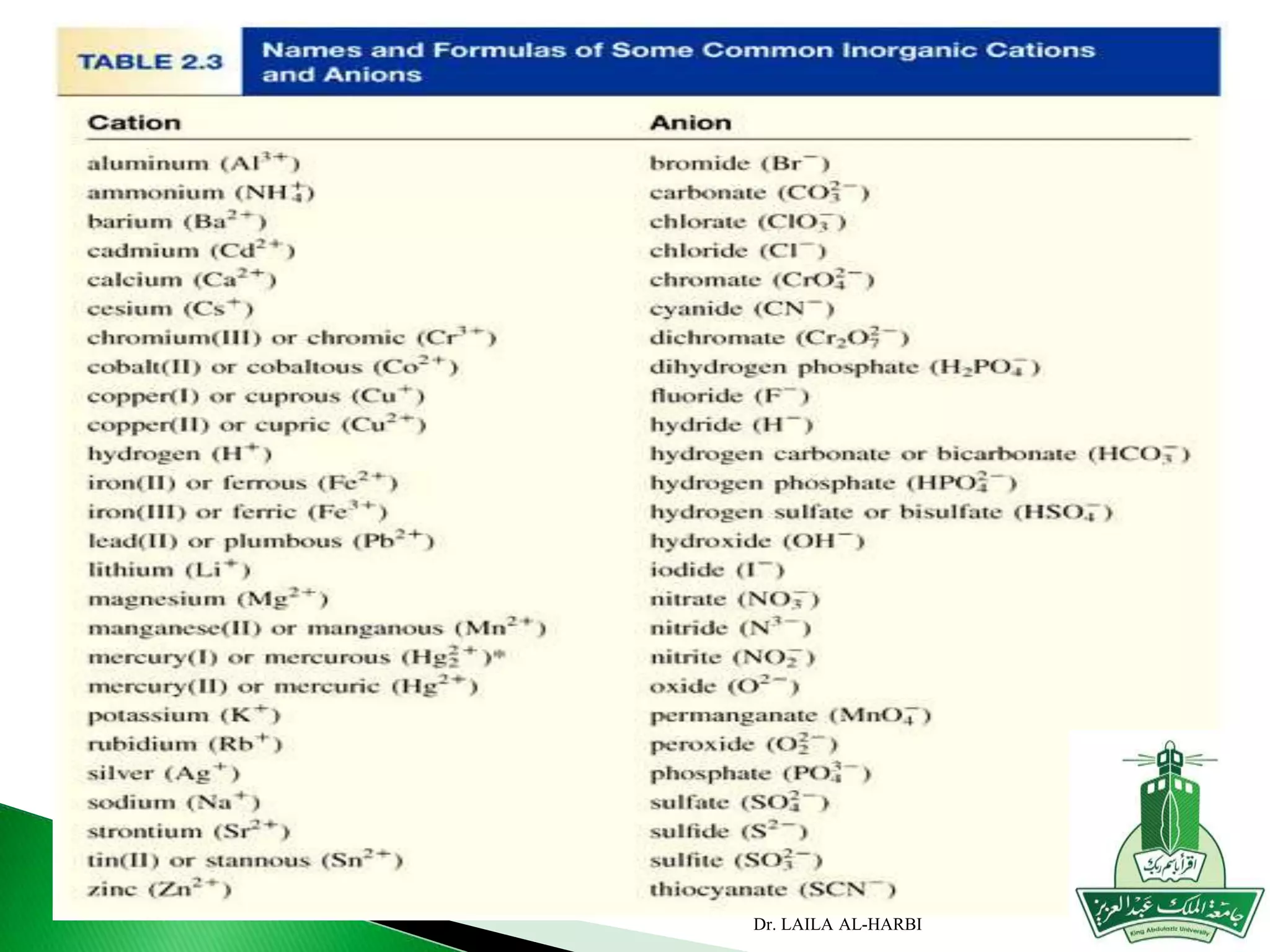
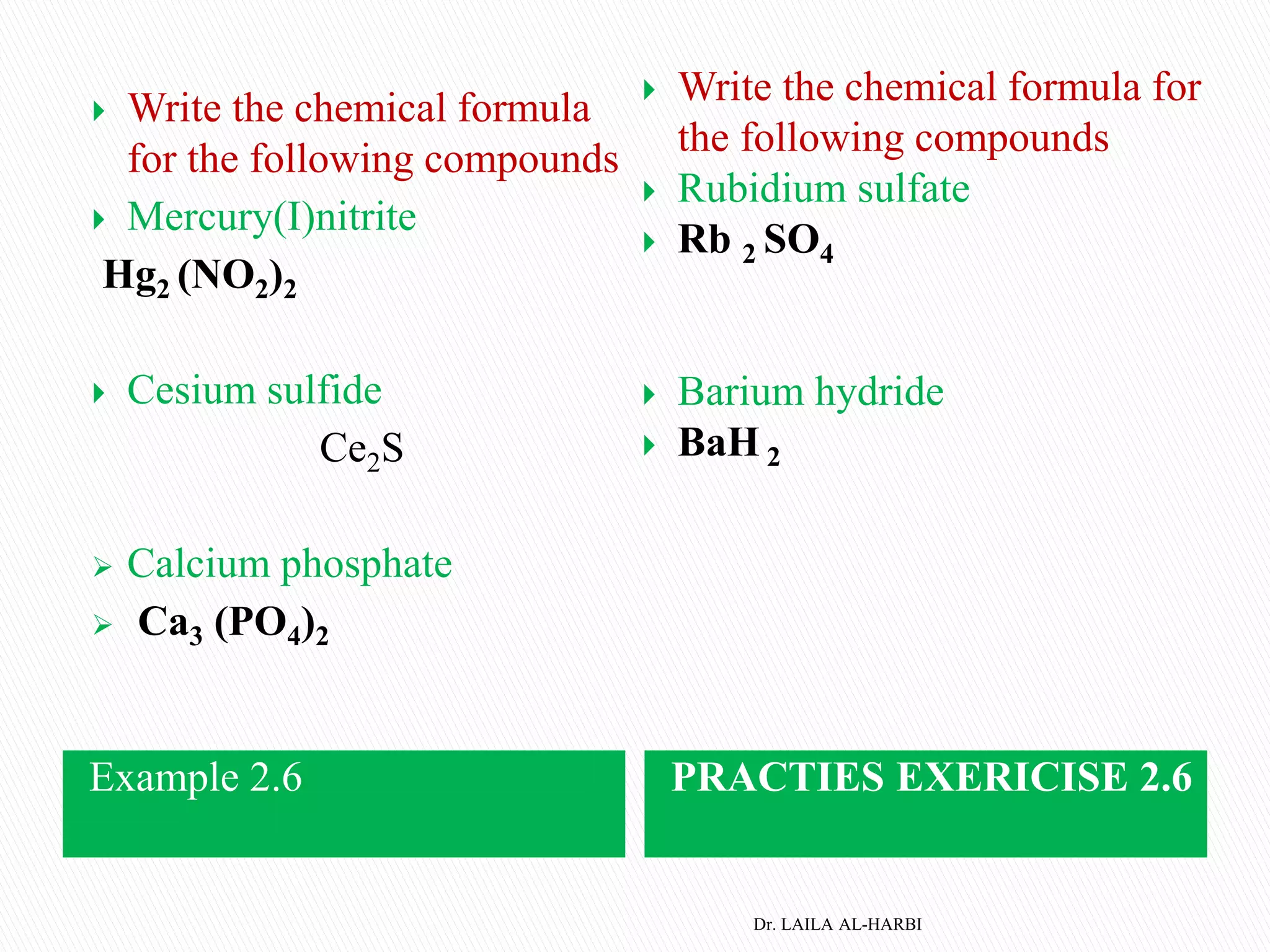
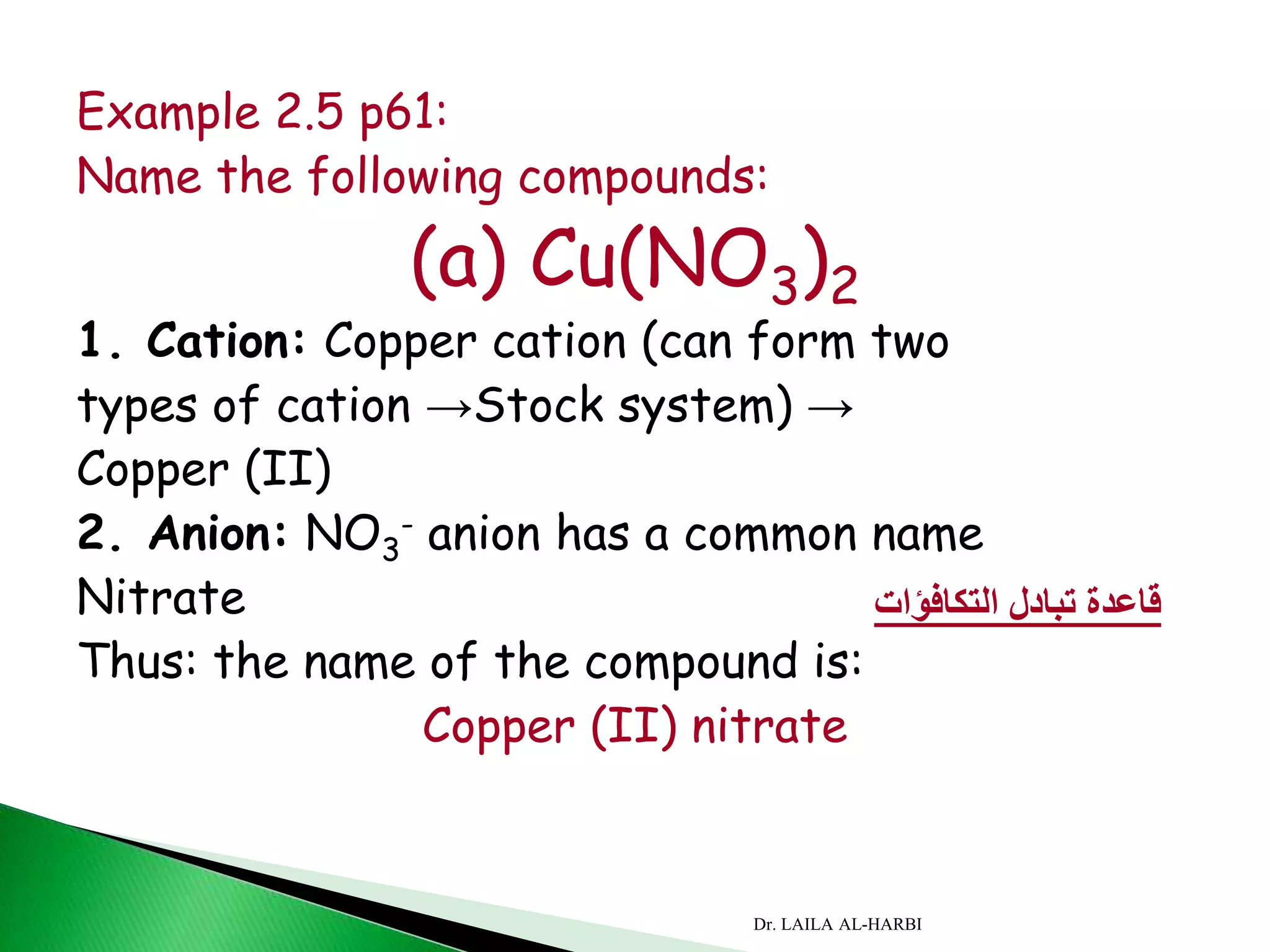
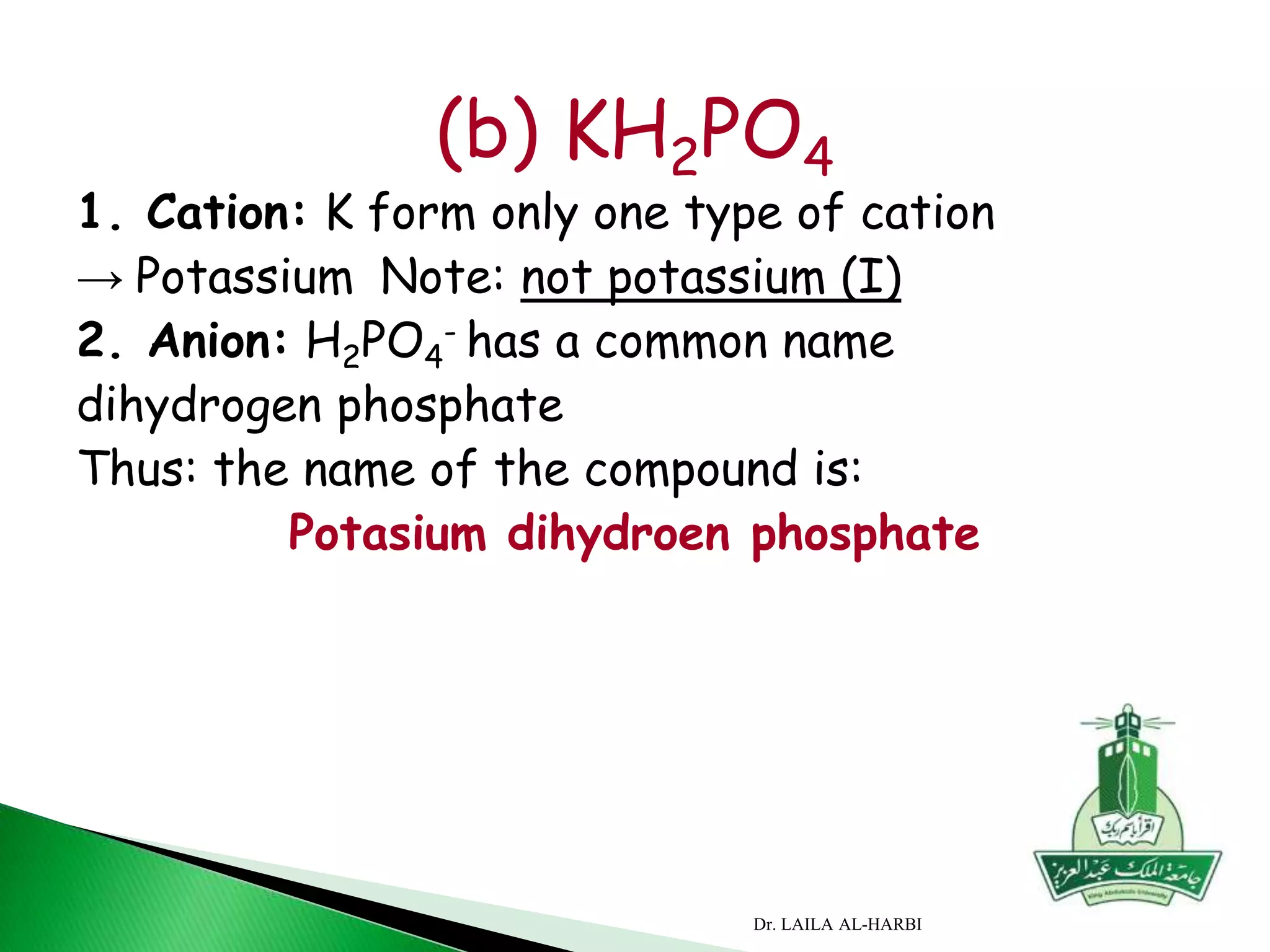
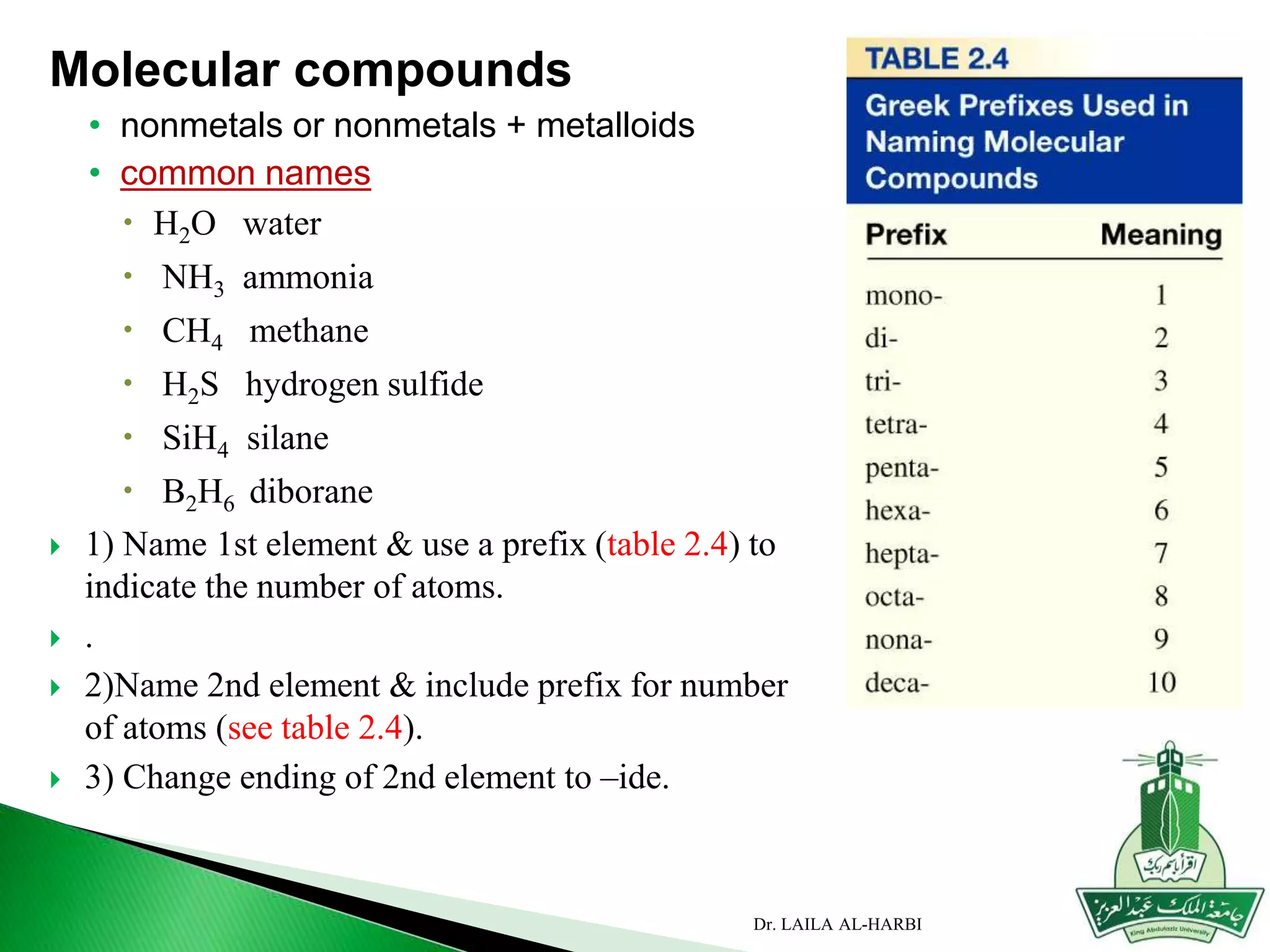
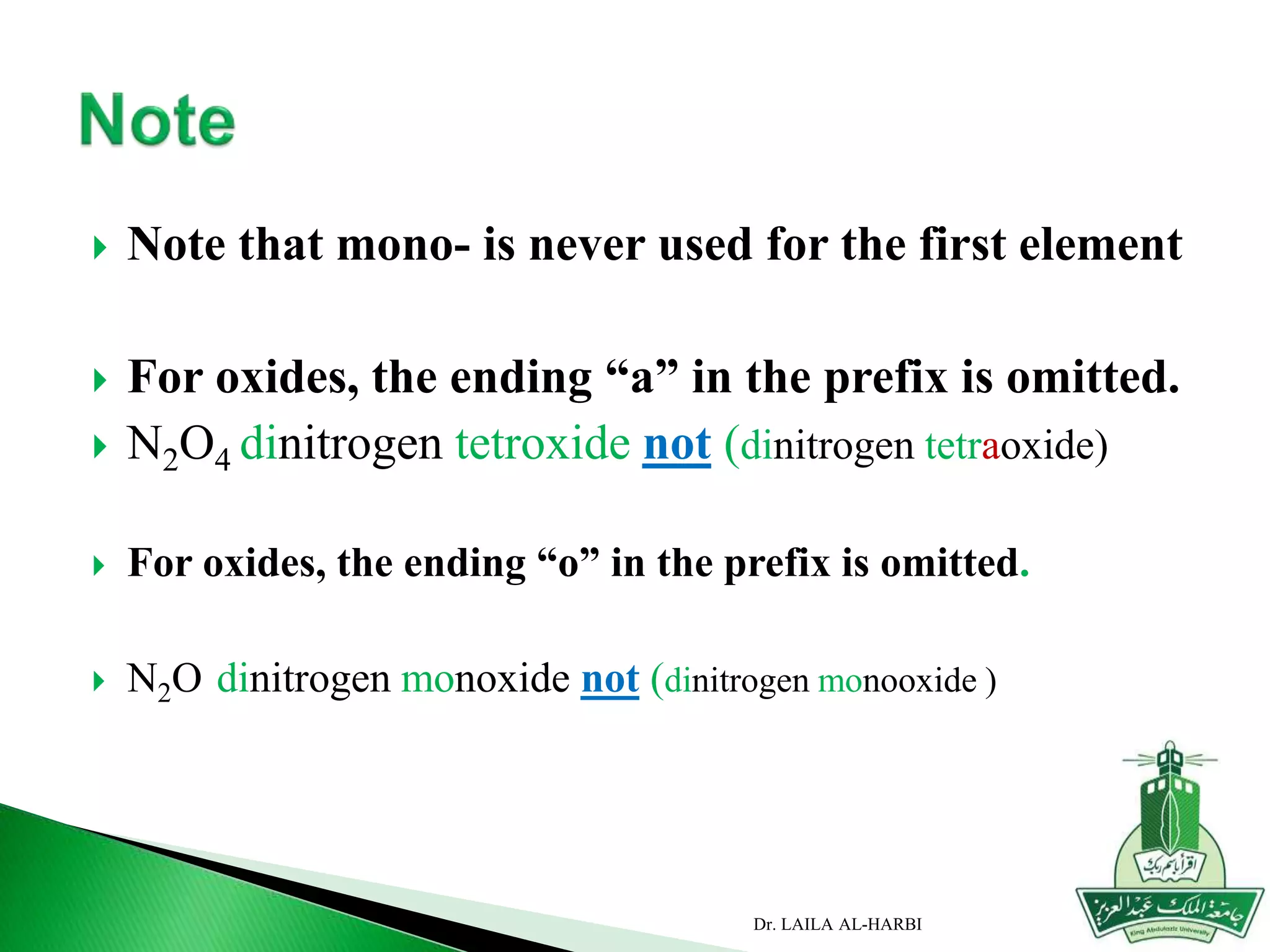
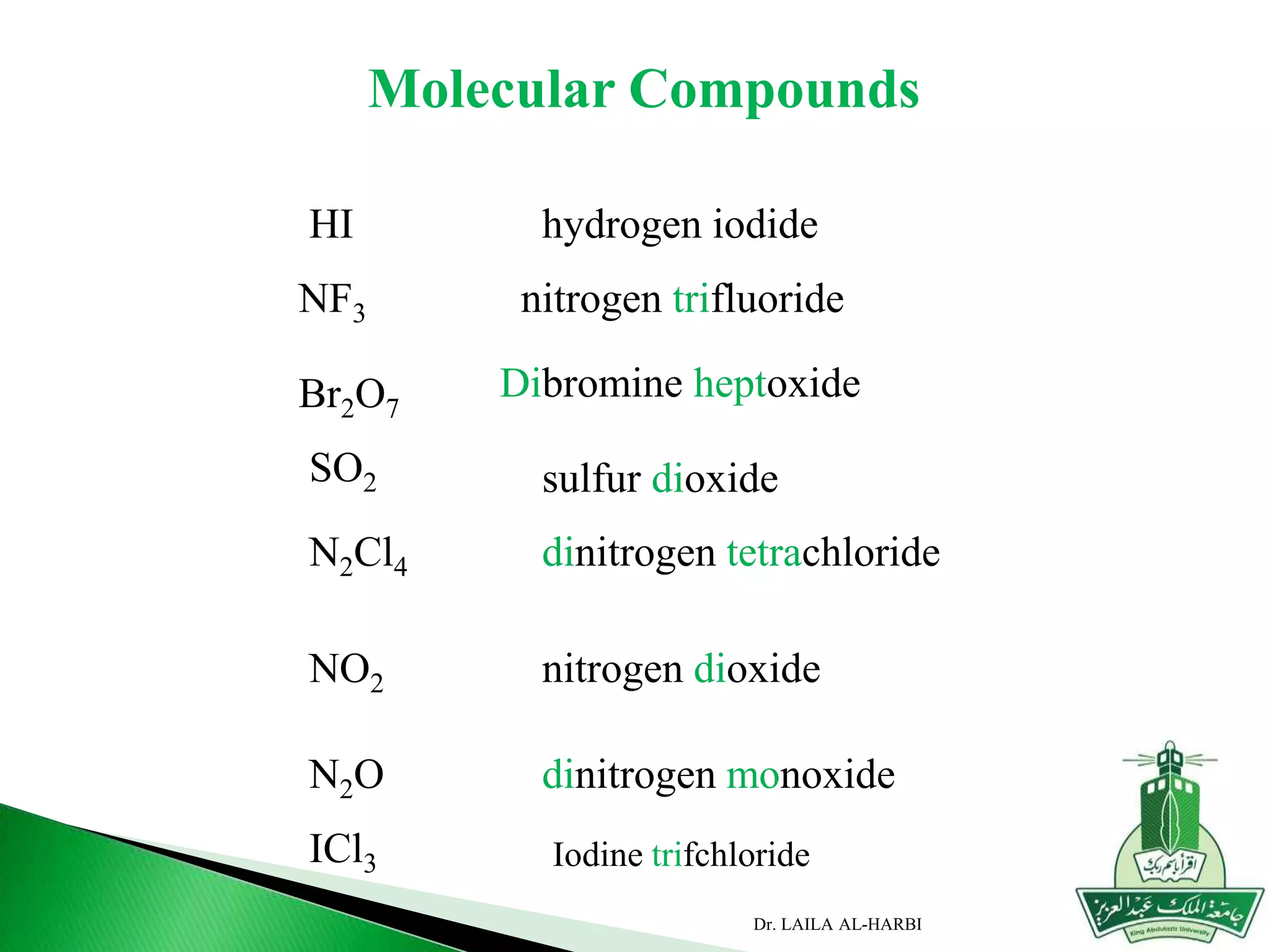
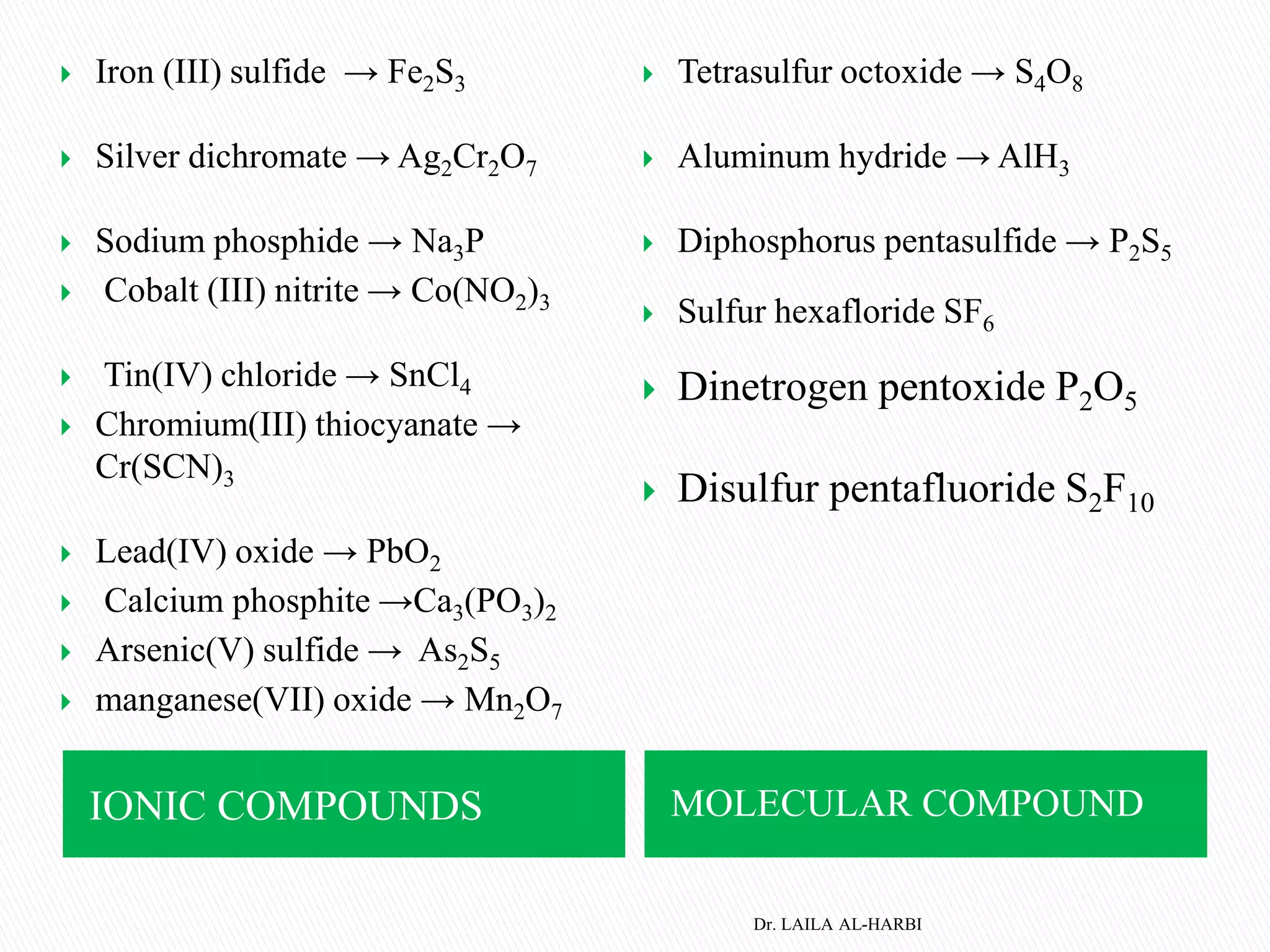
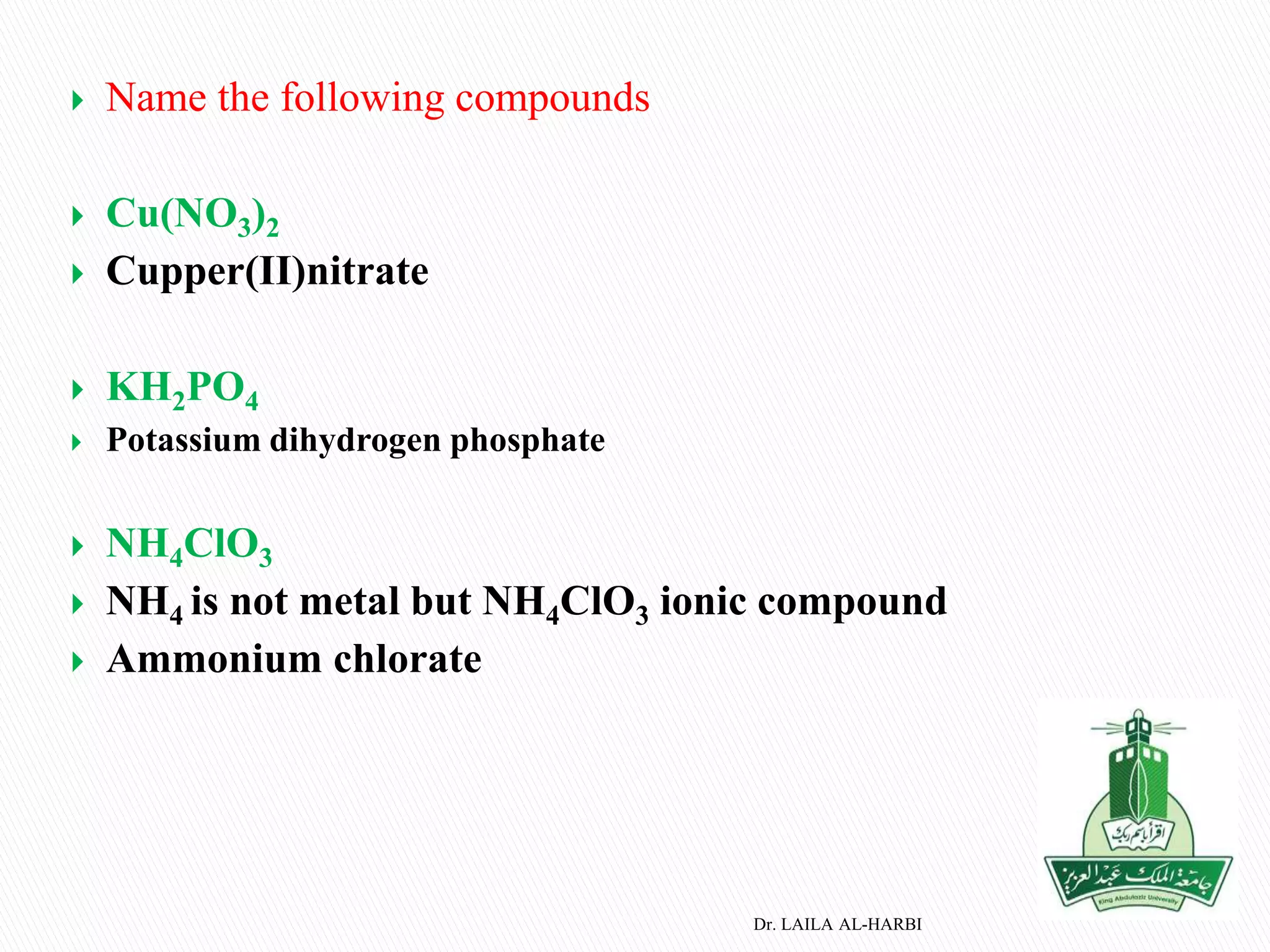
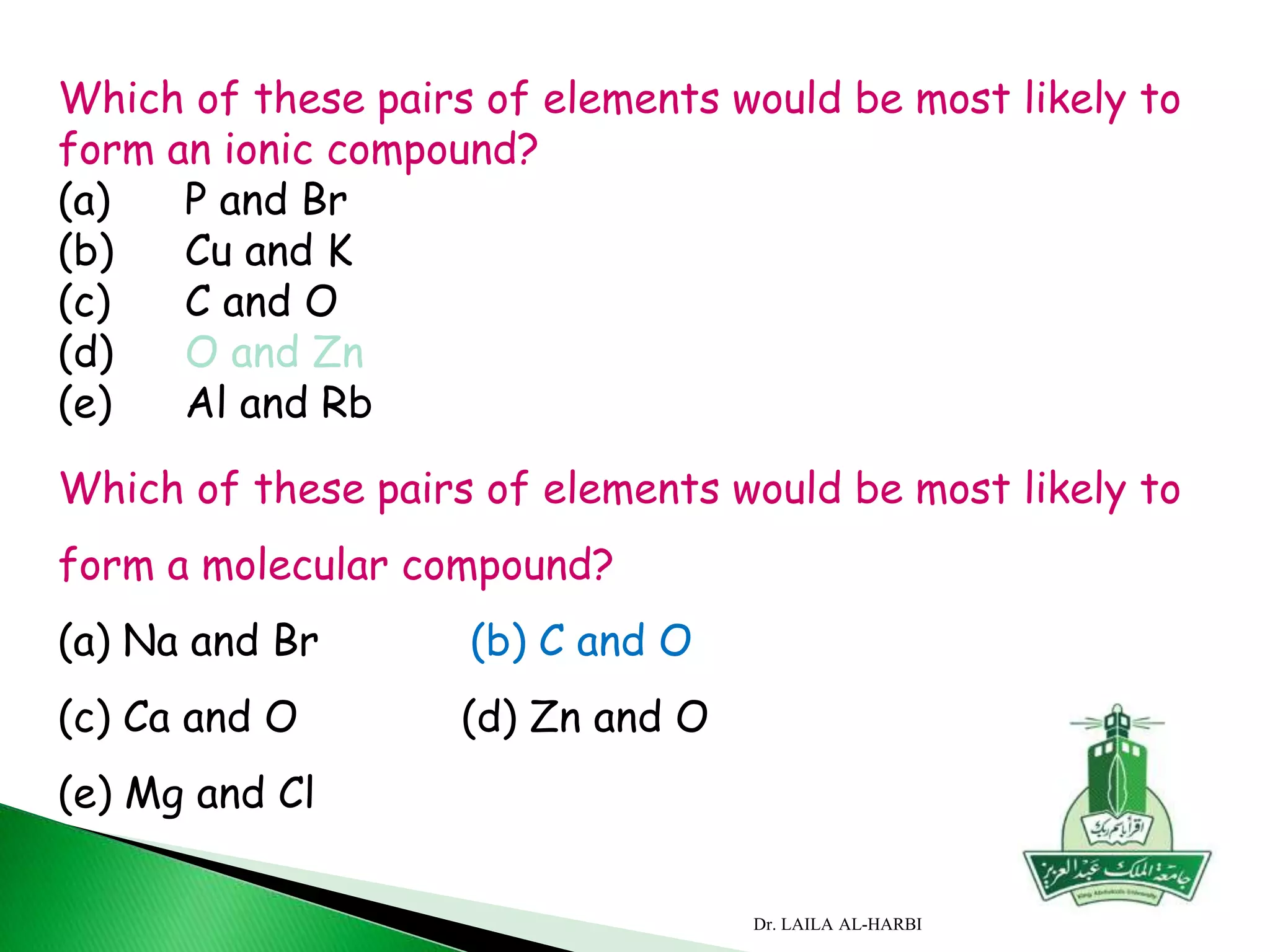
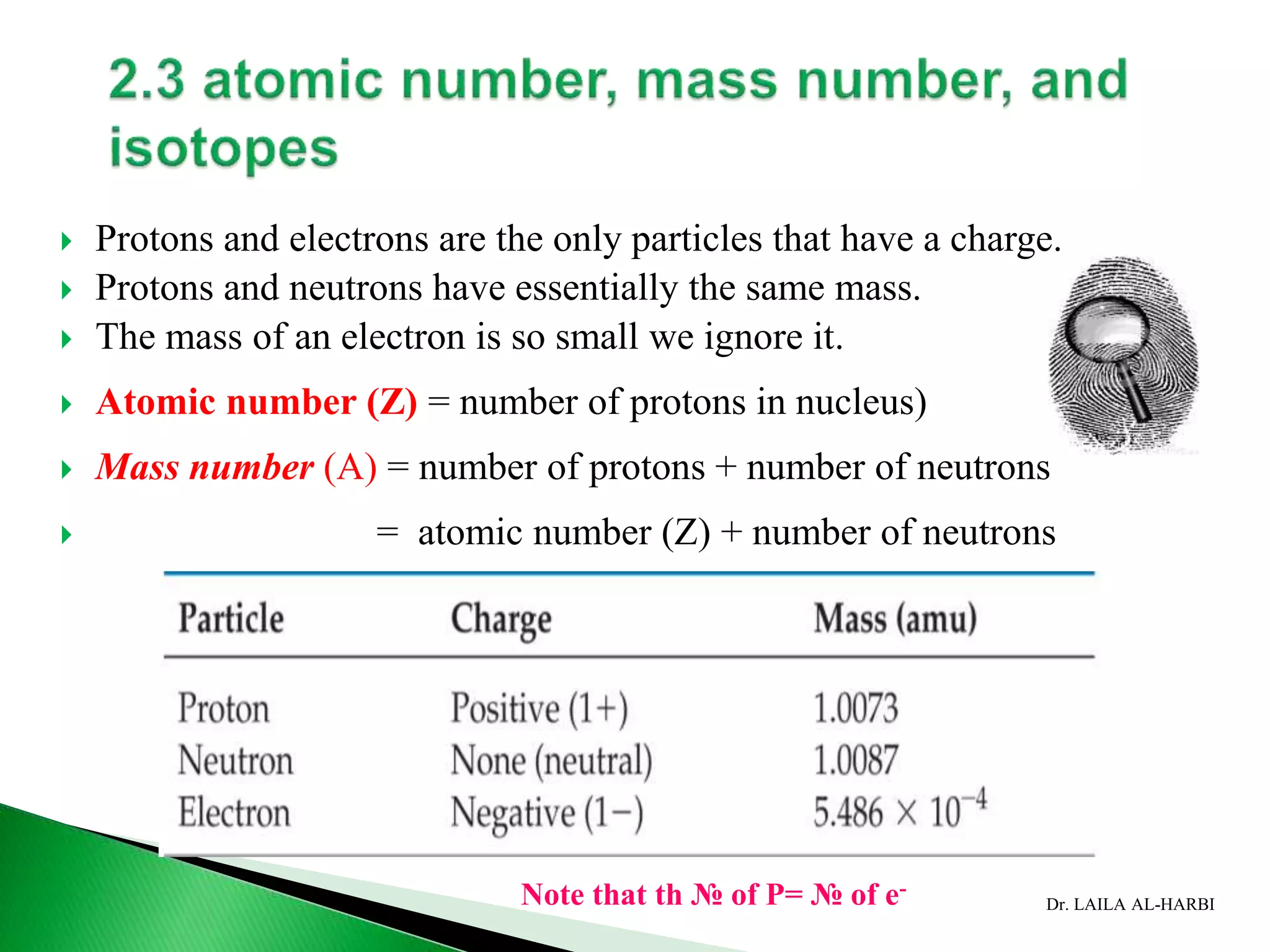
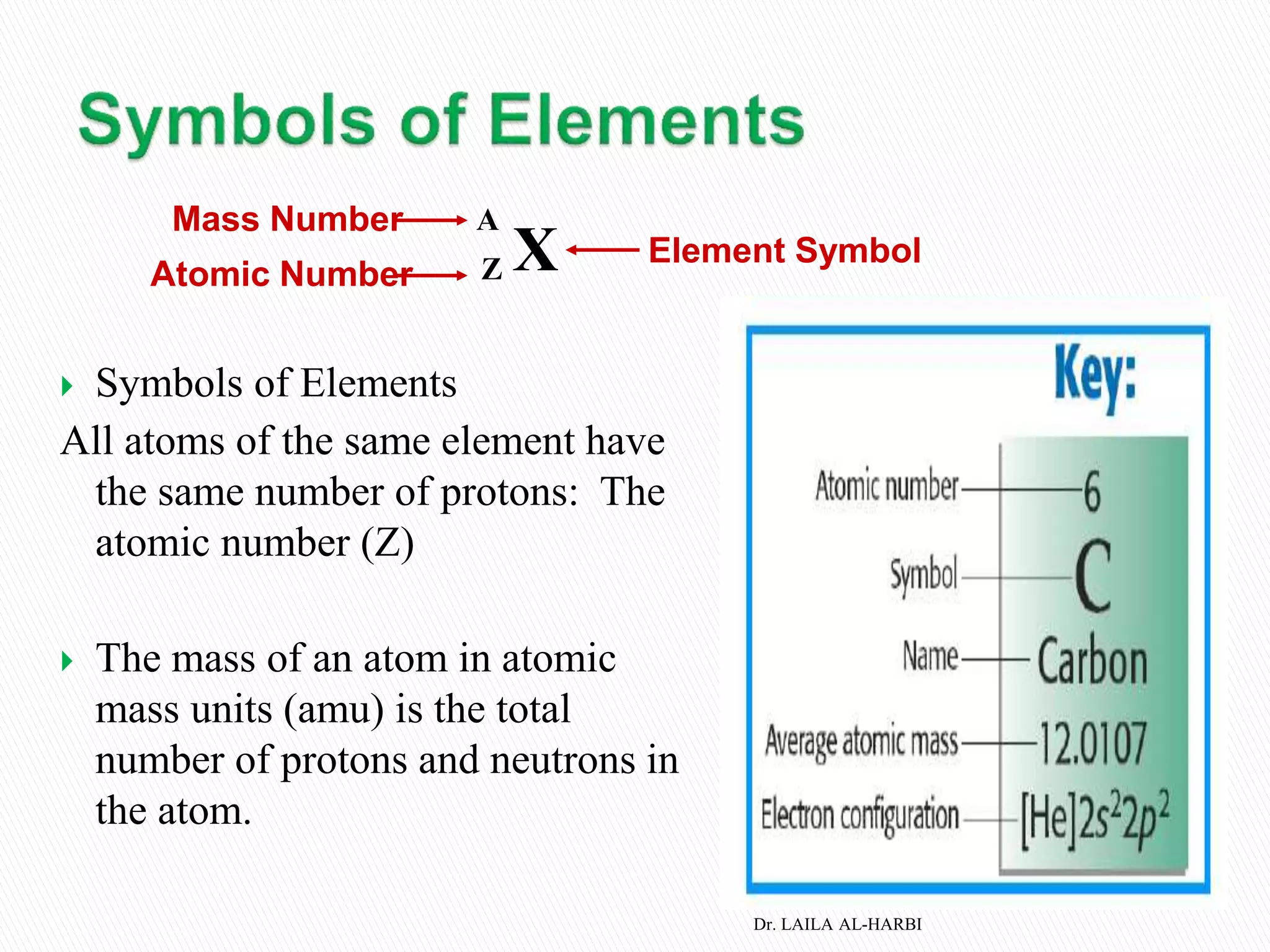
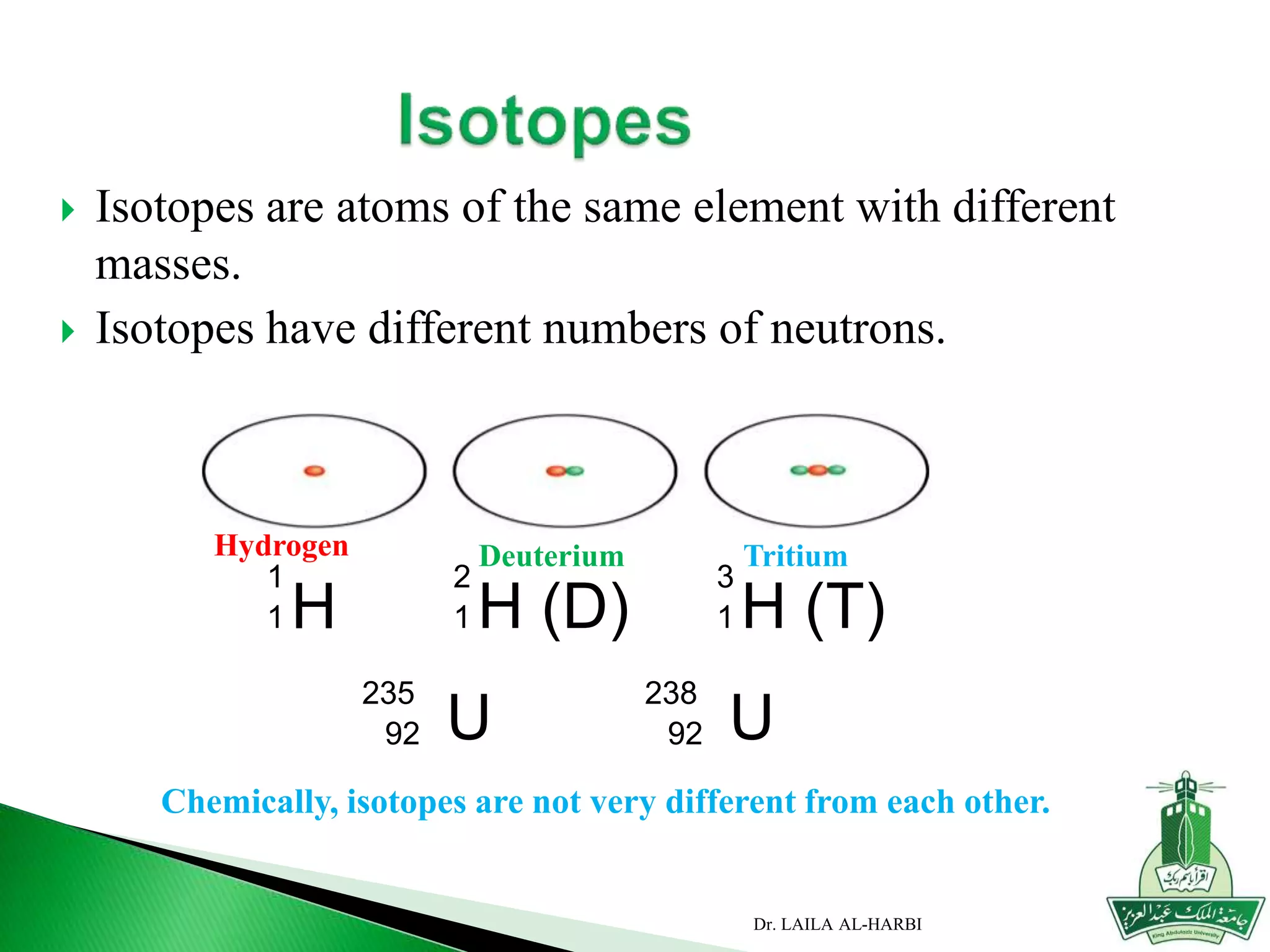
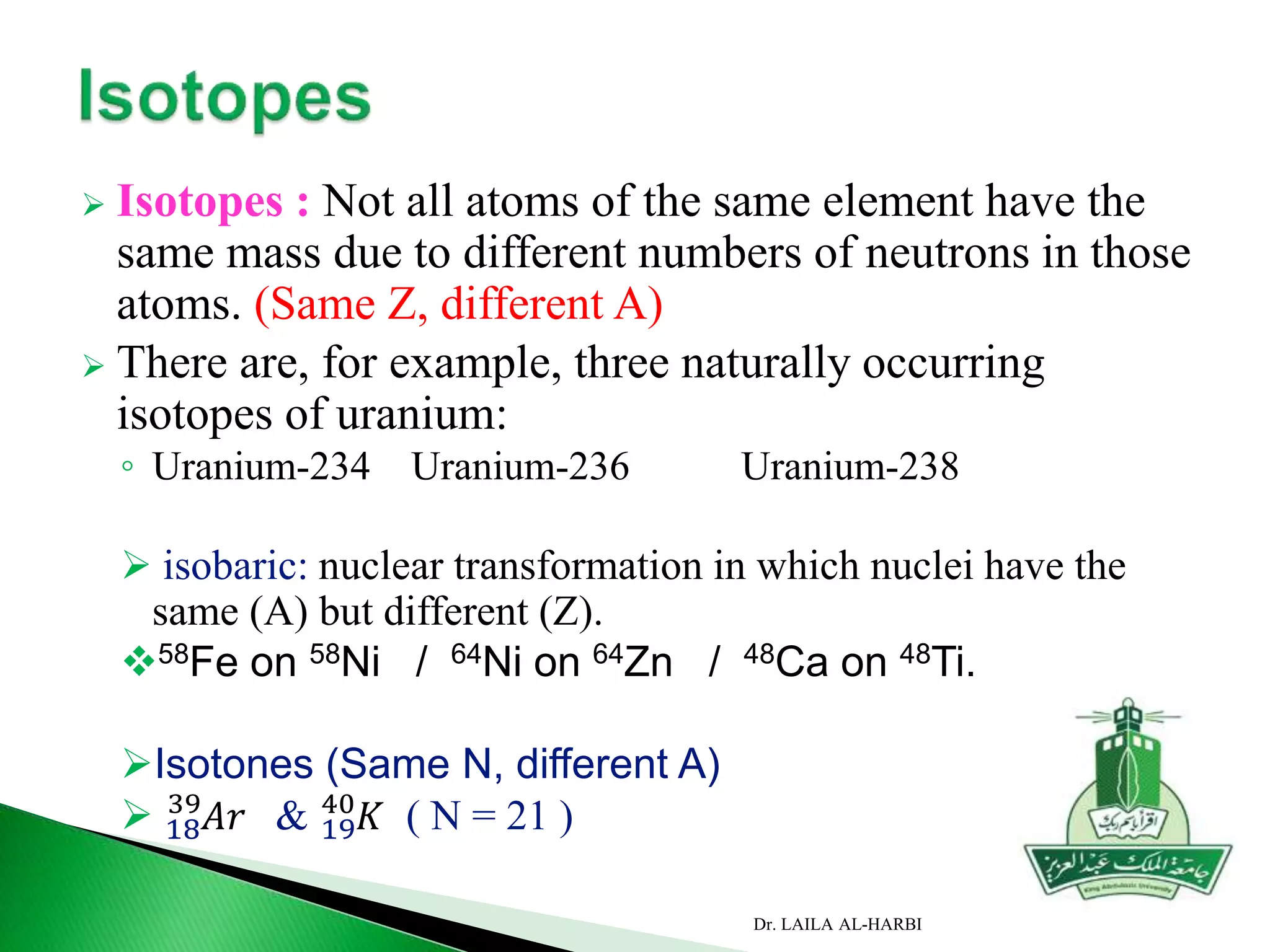
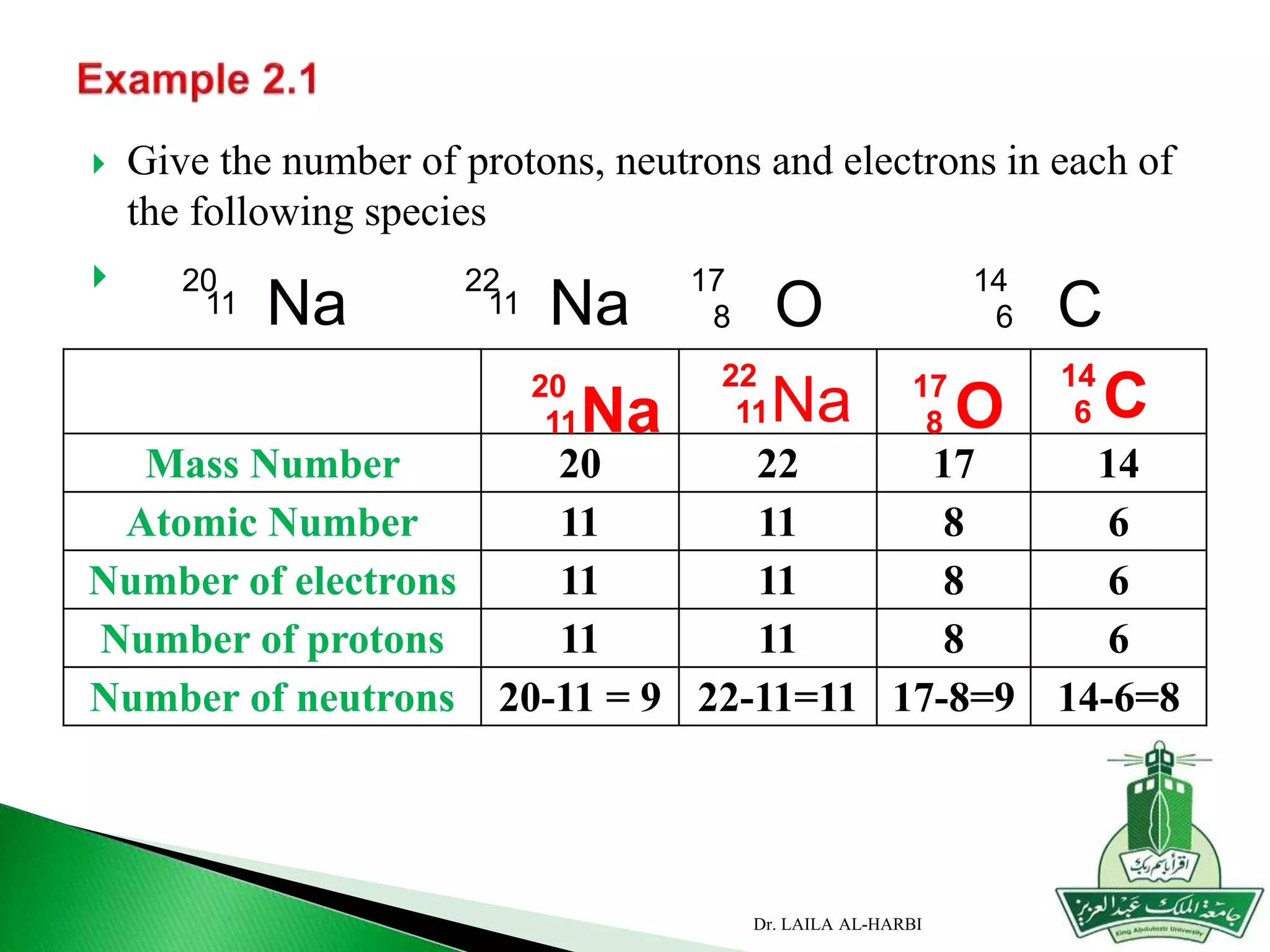
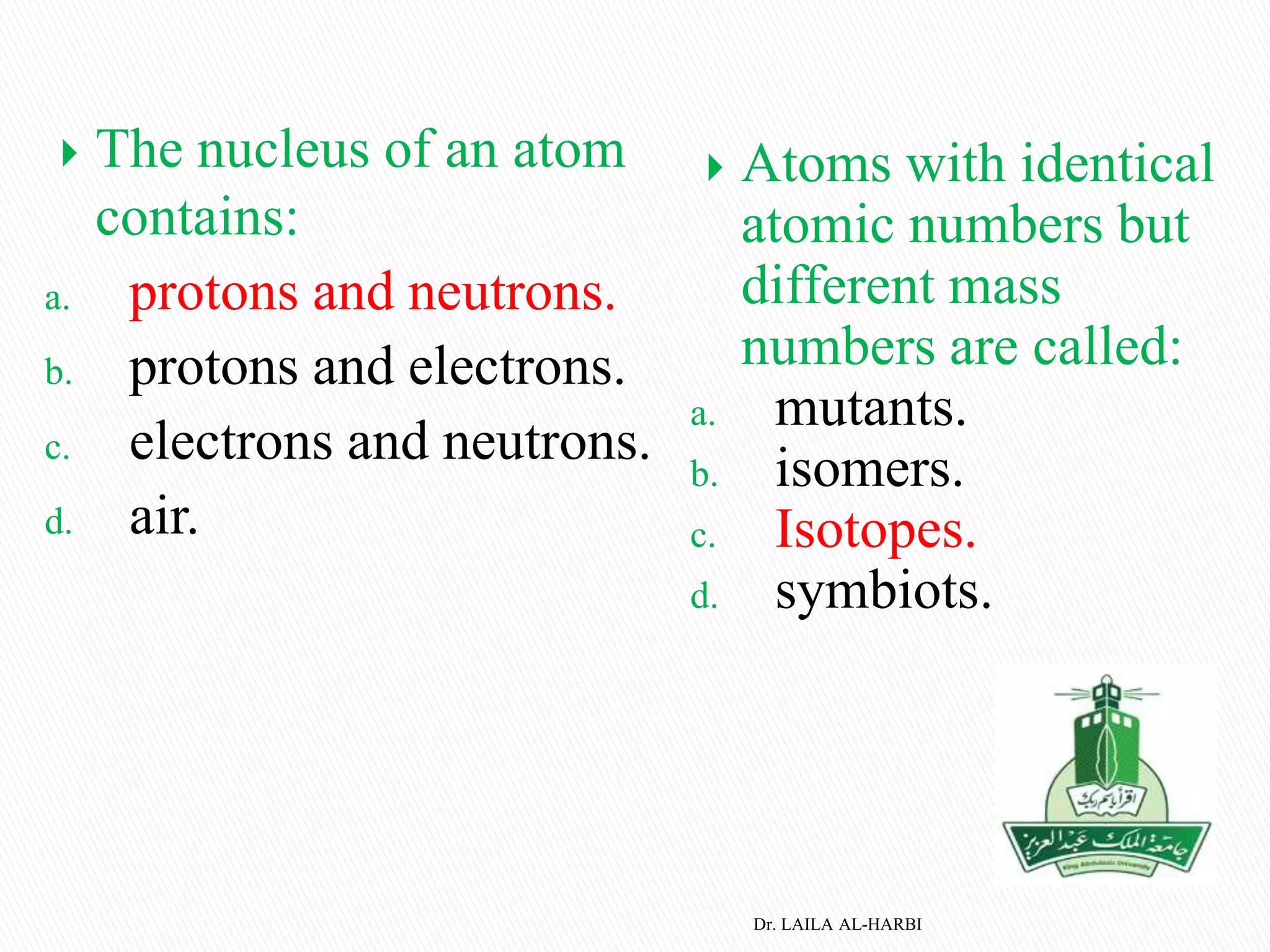
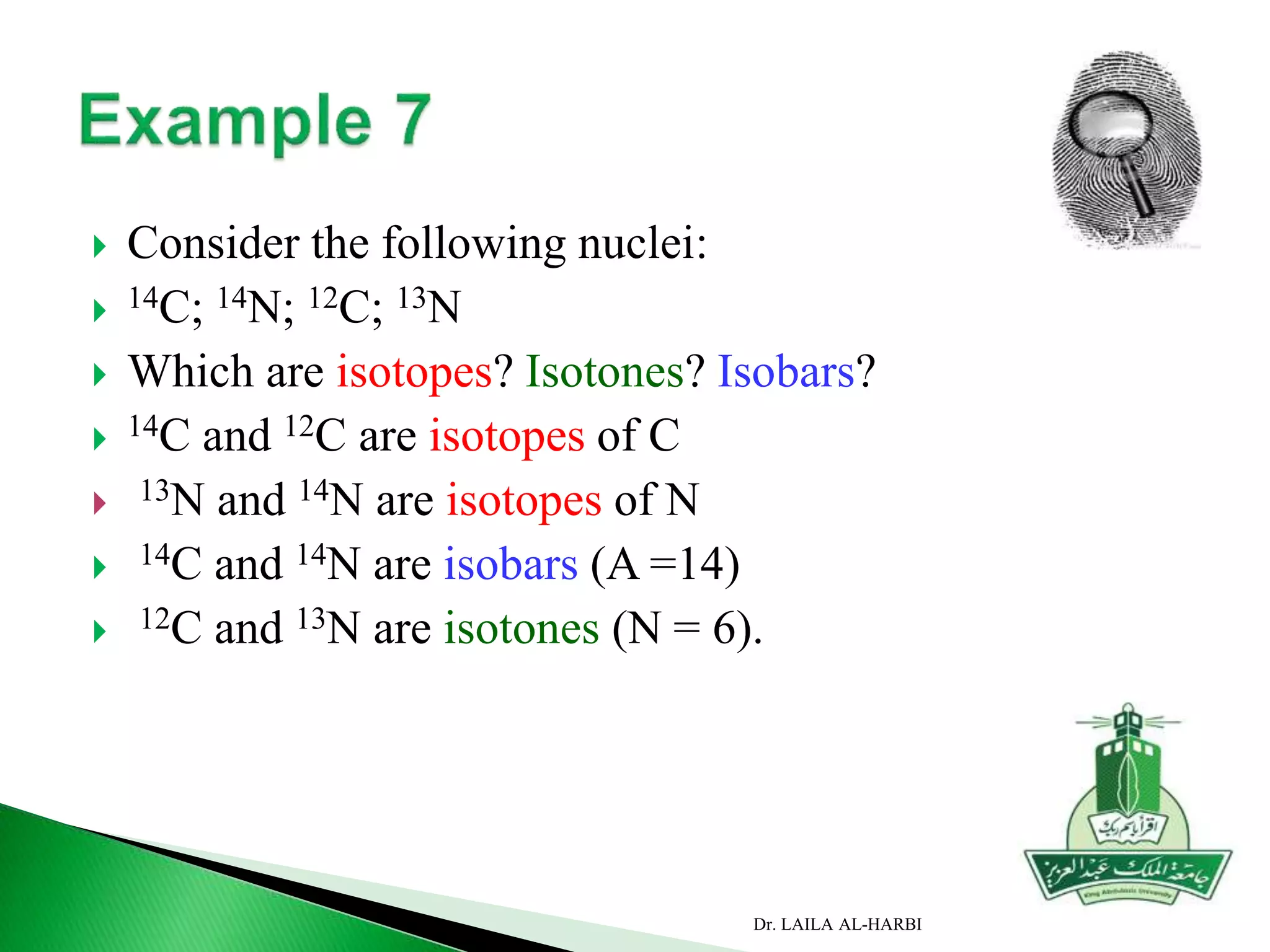
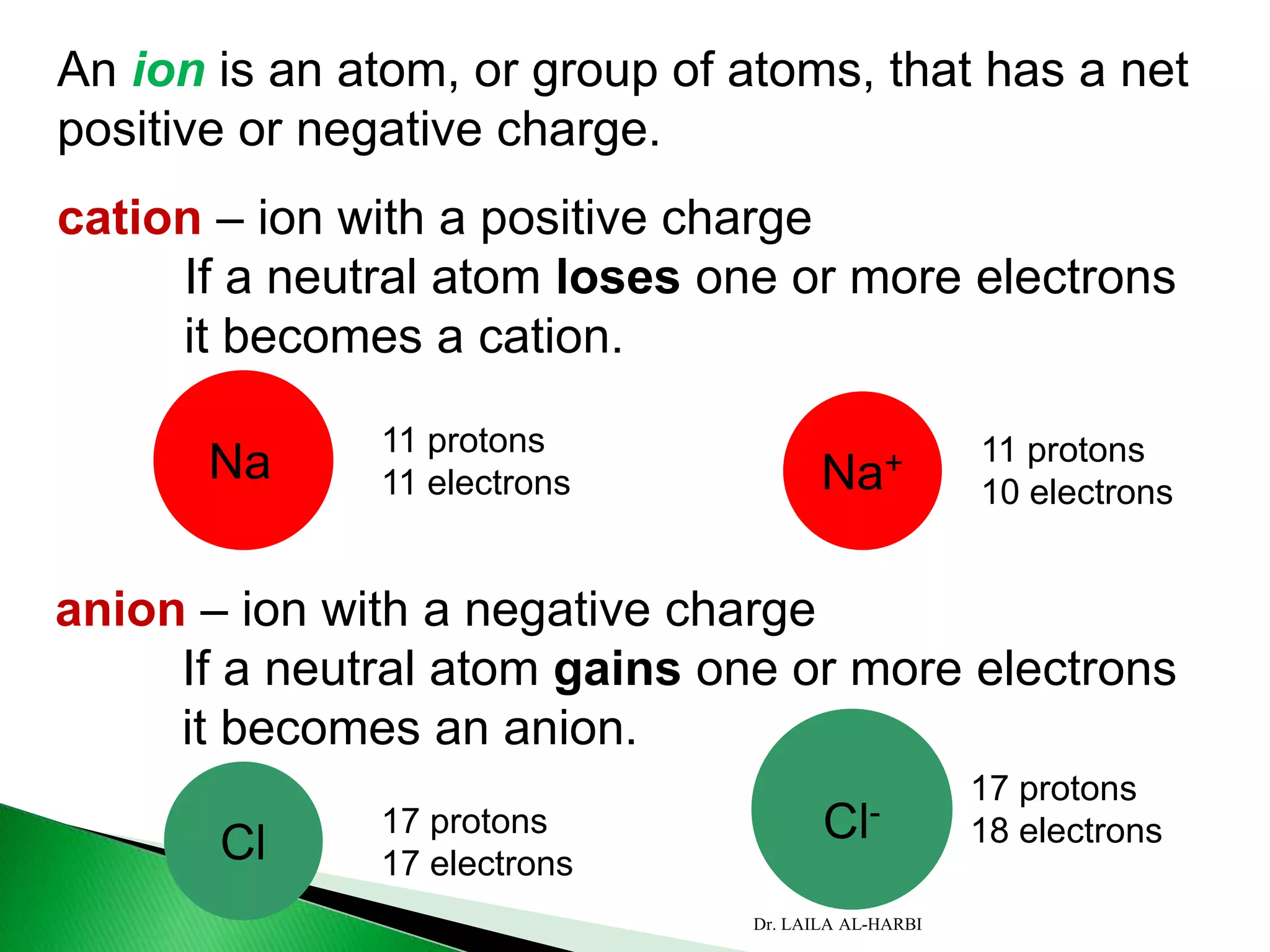
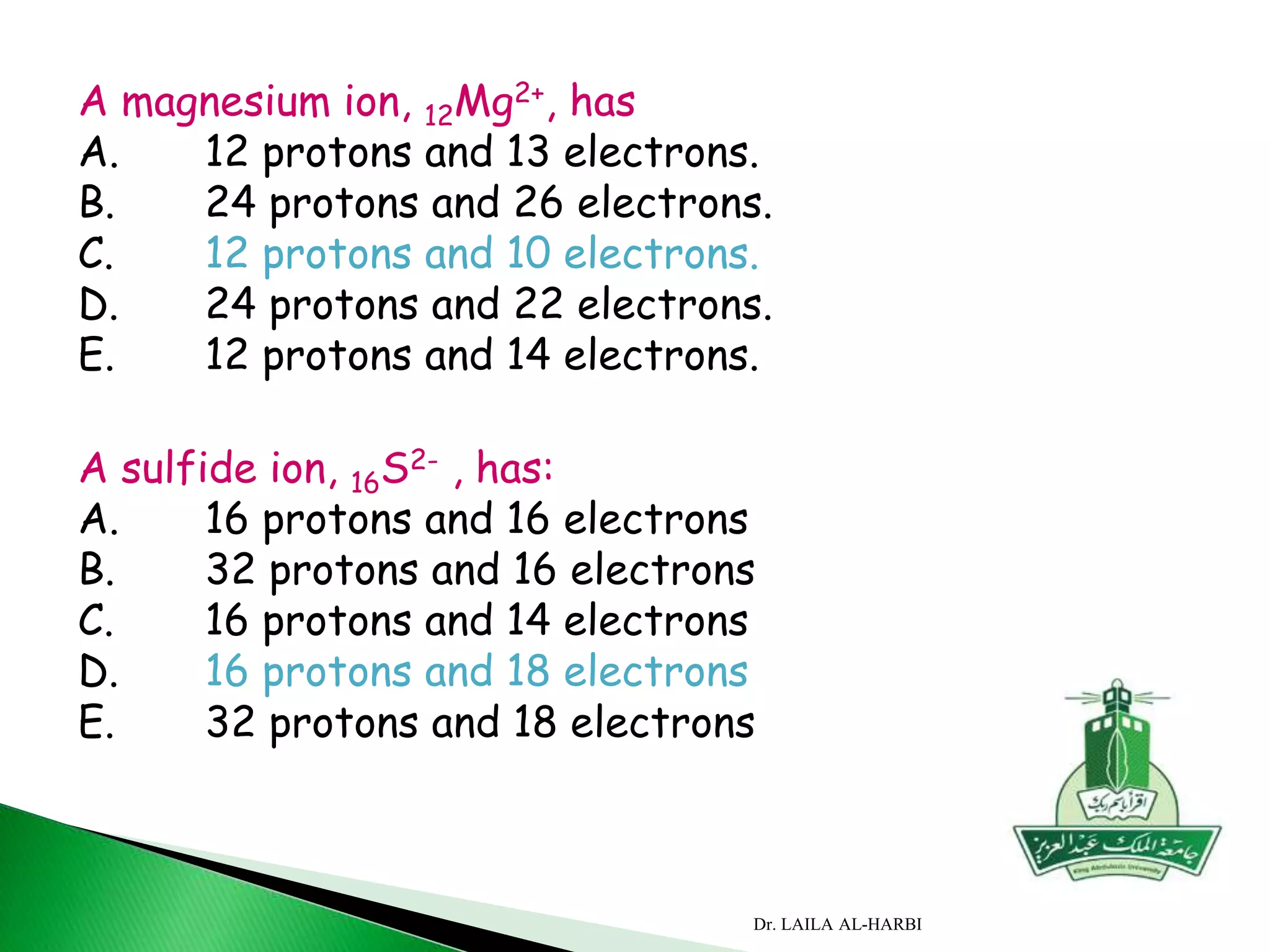
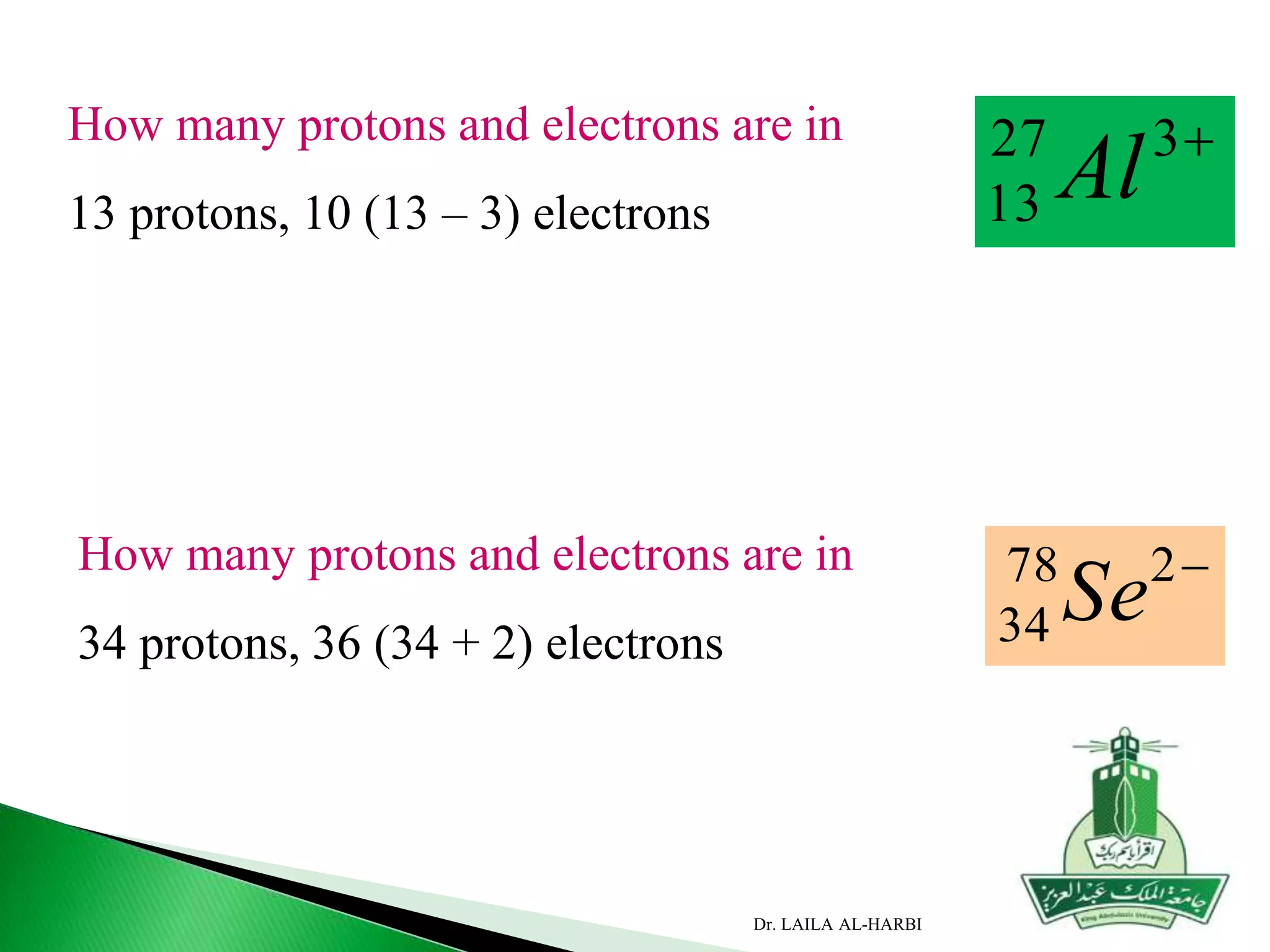
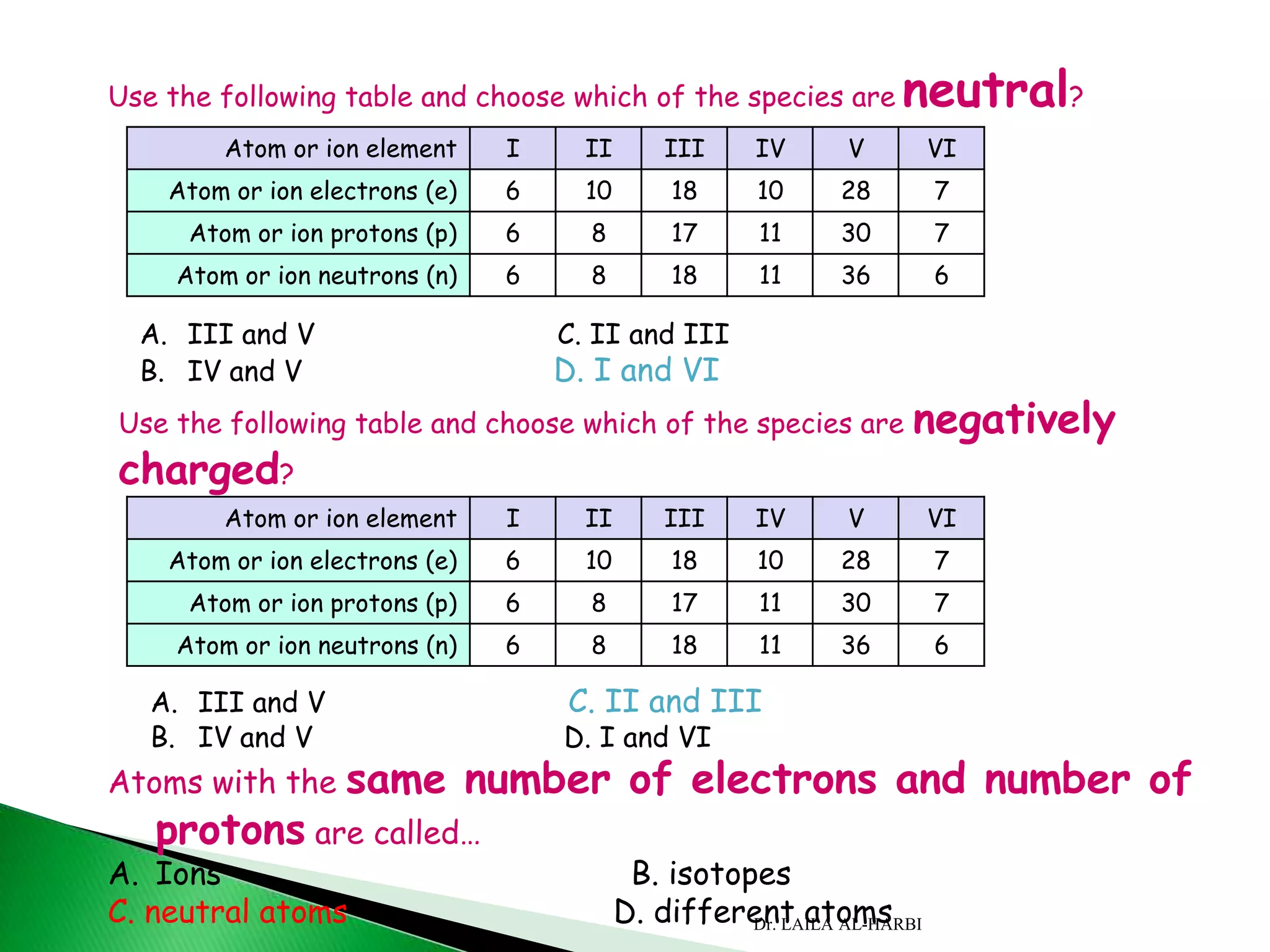
This document provides information about naming ionic and molecular compounds. It discusses atomic number, mass number, and isotopes. It explains that ions are formed when atoms gain or lose electrons to become positively or negatively charged. The document outlines rules for writing chemical formulas, including ionic formulas which involve balancing charges between cations and anions. It also discusses naming conventions for ionic compounds based on metal cations and nonmetal anions. The document concludes by stating that molecular compounds consist of nonmetals and do not involve ion formation.

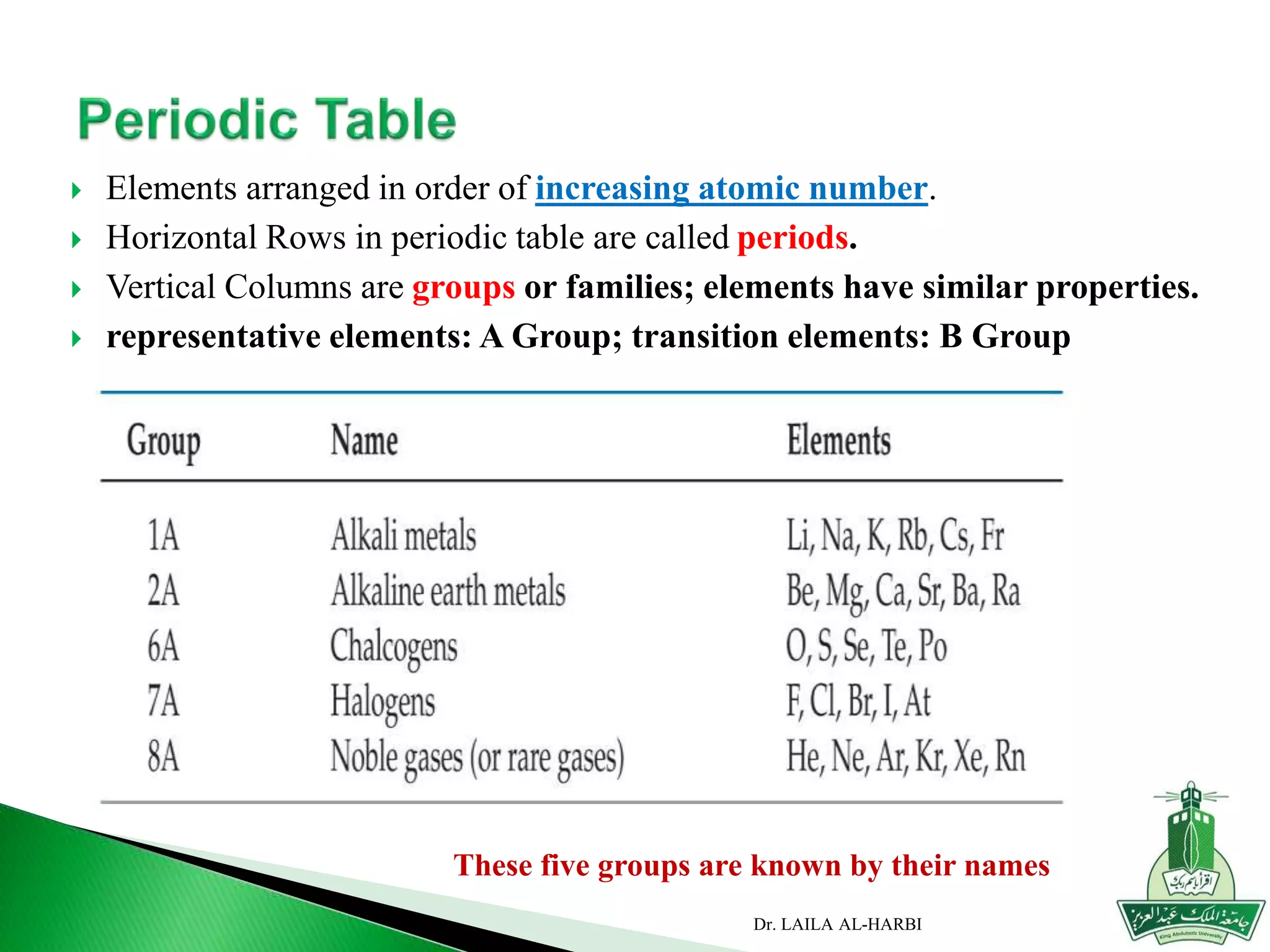
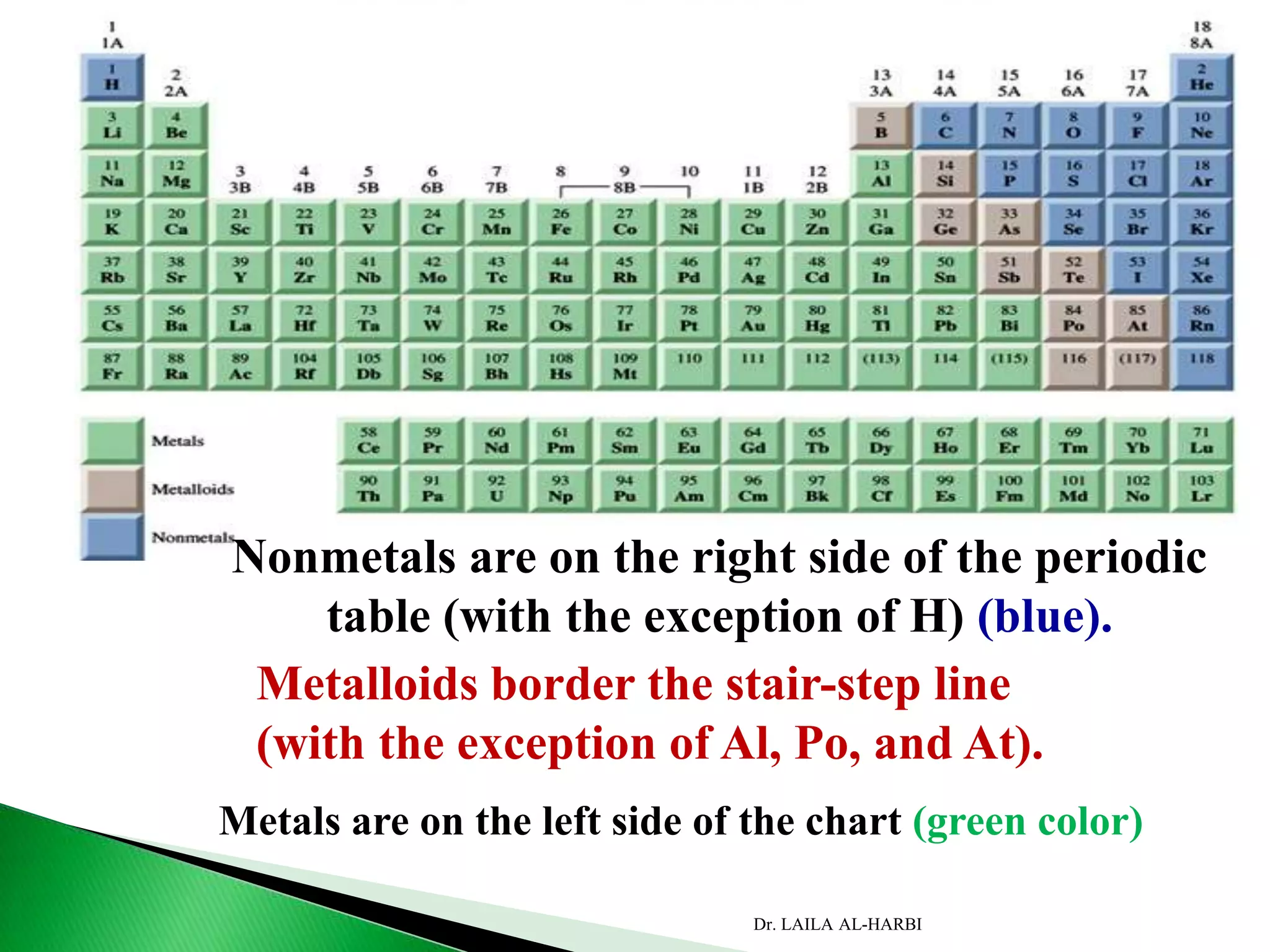
![Period
Group
AlkaliMetal
NobleGas
Halogen
AlkaliEarthMetal
Main-group elements [1A to 8A]
Transition metals
Dr. LAILA AL-HARBI](https://image.slidesharecdn.com/chapter2-141230110824-conversion-gate02/75/Chapter-2-15-2048.jpg)