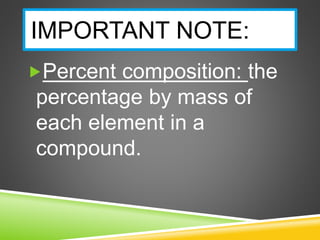
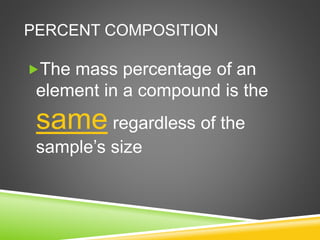
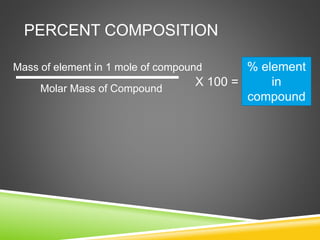
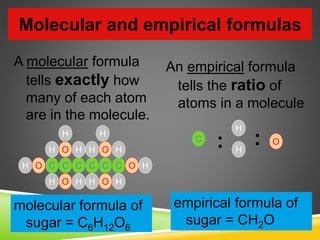
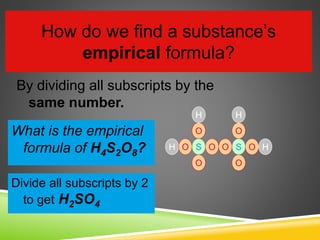
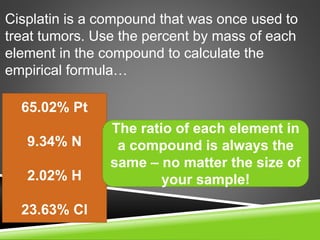
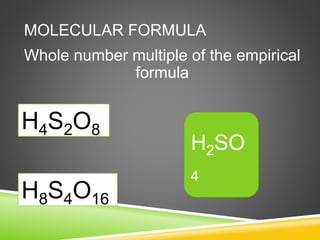
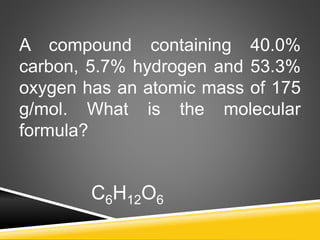
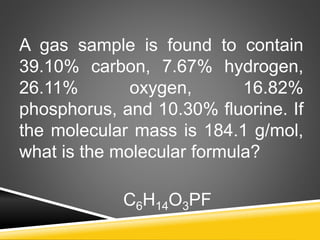
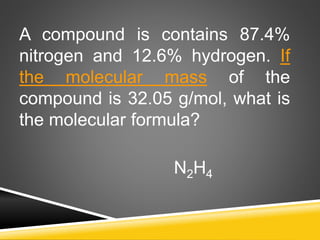
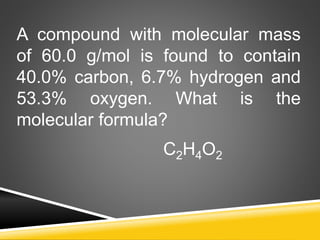
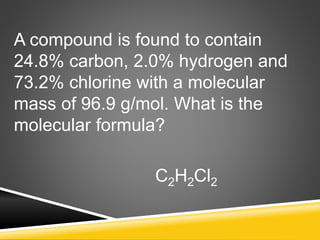
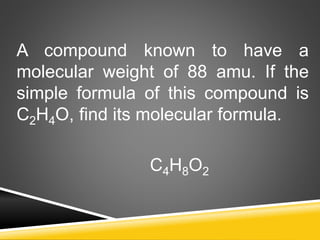
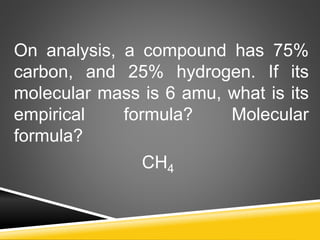
This document provides examples and explanations for calculating percent composition, empirical formulas, and molecular formulas. It begins with examples of calculating percent composition of elements in compounds and mixtures. It then defines empirical and molecular formulas, and provides steps for calculating empirical formulas from mass percentages of elements or experimental data. Several examples are worked through. The document emphasizes that empirical formulas show the simplest whole number ratio of elements in a compound, while molecular formulas indicate the actual number of each type of atom in a molecule.