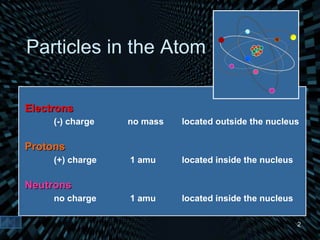
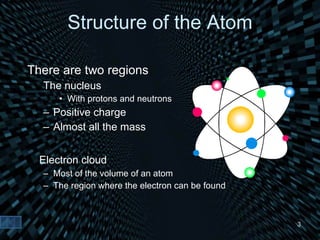
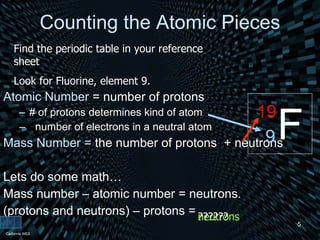
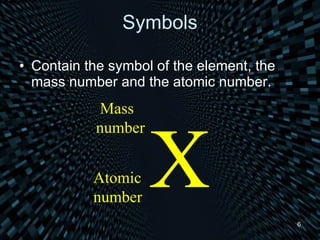
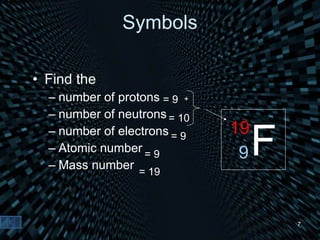
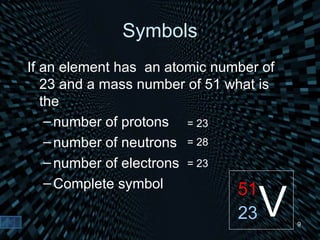
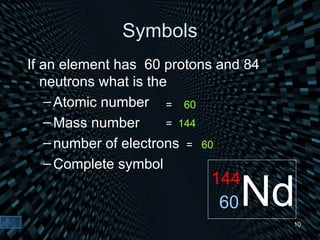
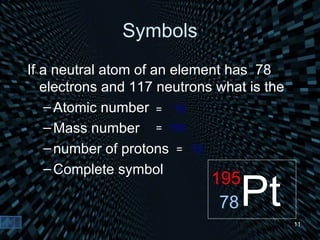
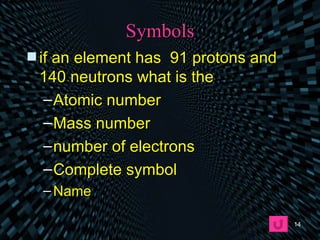
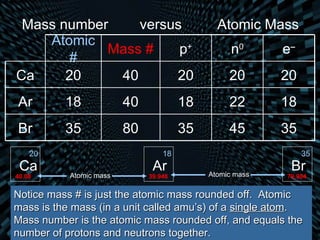
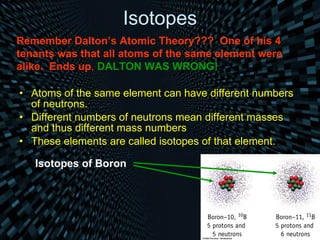
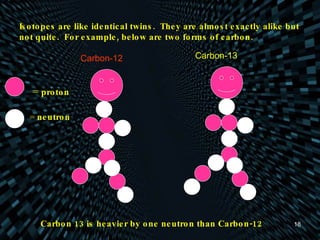
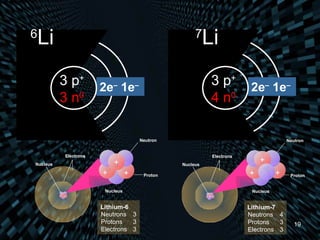
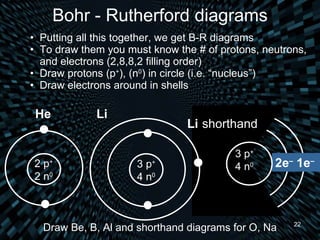
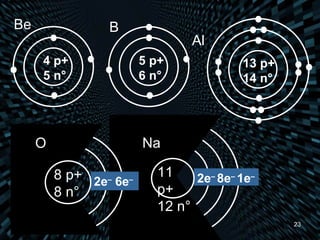
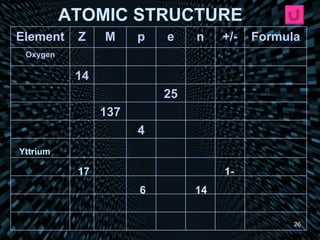
Each element is unique due to its atomic structure. Atoms consist of protons and neutrons located in the nucleus, and electrons outside the nucleus. The number of protons determines the element, while the total of protons and neutrons is the mass number. Isotopes are atoms of the same element with different numbers of neutrons.