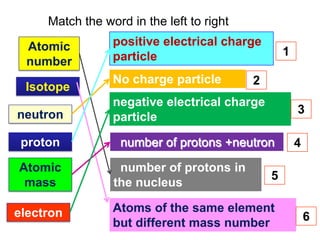
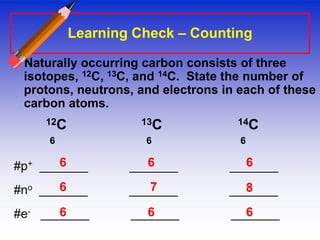
The document provides information about the fundamental particles that make up atoms, including protons, neutrons, electrons, and isotopes. It defines key atomic properties such as atomic number, which is the number of protons in an atom's nucleus, and mass number, which is the total number of protons and neutrons. It notes that atoms of the same element can have different numbers of neutrons, making them isotopes. As an example, it provides the particle makeup of several carbon isotopes: 12C, 13C, and 14C.