Embed presentation
Downloaded 27 times
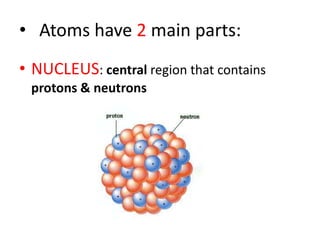
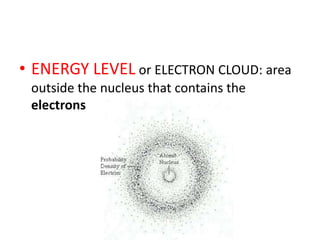
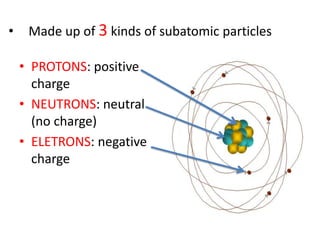
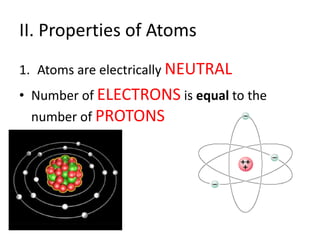
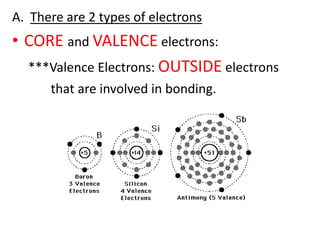

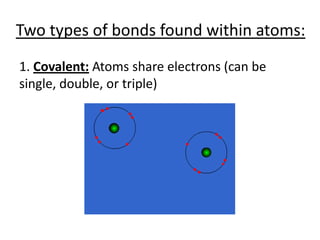
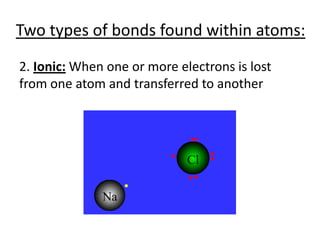

Matter is made up of atoms, which contain a nucleus of protons and neutrons surrounded by an electron cloud. Atoms are electrically neutral and bond with other atoms by sharing, losing, or gaining electrons in their outer valence shell. There are two main types of bonds: covalent bonds form when atoms share electrons, and ionic bonds form when one atom transfers an electron to another. Understanding the basic building blocks of matter and how atoms bond is fundamental to chemistry.










