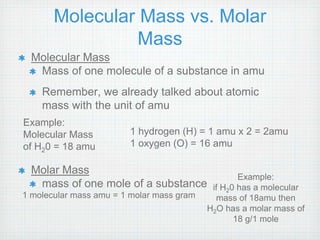
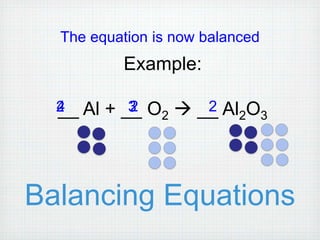
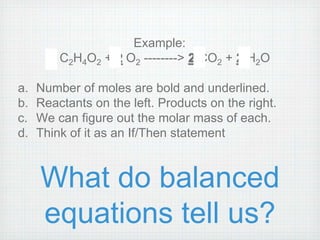
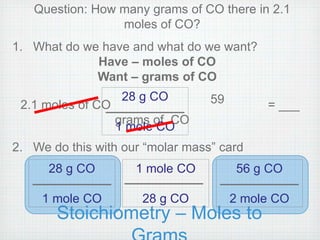
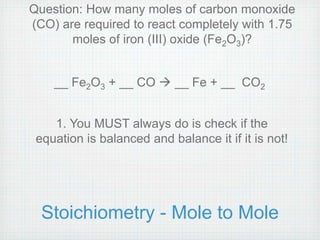
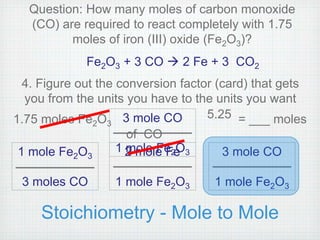
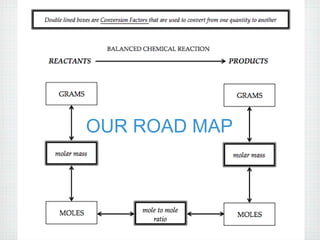
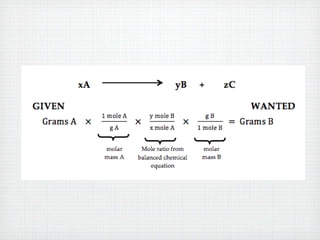
This document provides an overview of moles, molar mass, and stoichiometry. It defines a mole as 6.02x1023 particles of an element or compound. Avogadro's number is equal to this quantity of particles. Molecular mass is the mass of one molecule in atomic mass units (amu) while molar mass is the mass of one mole of a substance in grams. Examples are given for calculating molecular and molar mass. The document then discusses balancing chemical equations and using molar ratios from balanced equations to solve stoichiometry problems involving moles, grams, and particles of reactants and products.