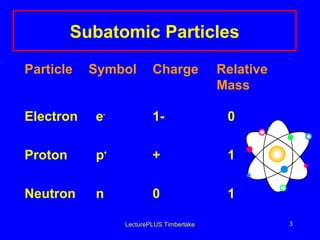
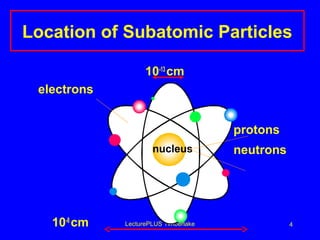
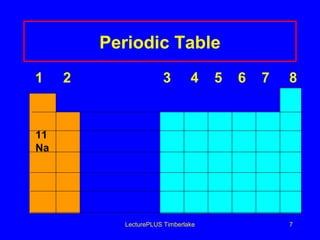
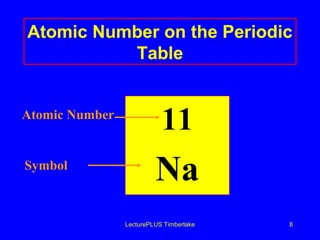
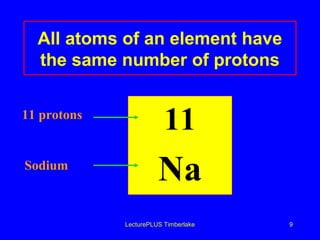
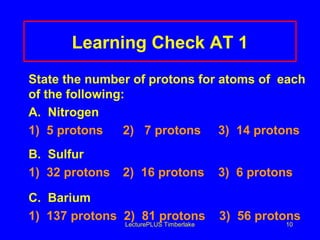
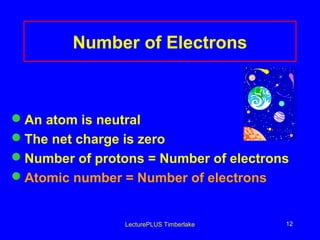
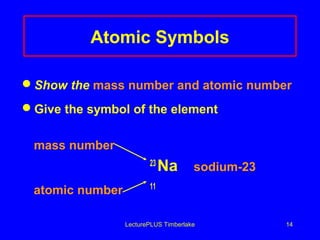
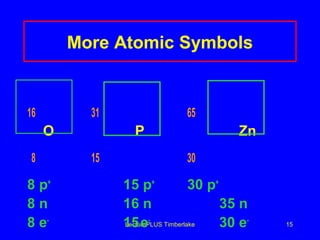
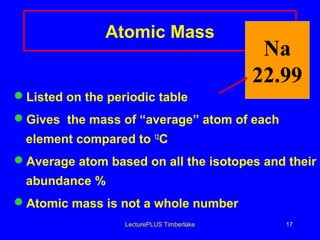
This document discusses the structure of atoms including subatomic particles like protons, neutrons, and electrons. It explains key atomic concepts such as atomic number, mass number, isotopes, and the periodic table. The periodic table arranges elements by atomic number and can be used to find protons, electrons, atomic symbols, and average atomic masses. Atoms of the same element have the same number of protons.