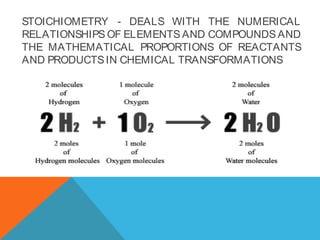
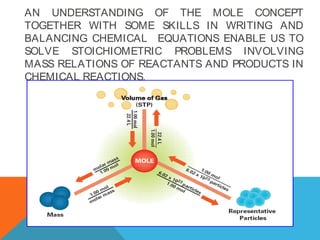
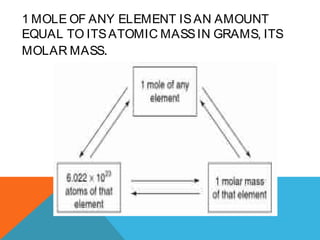
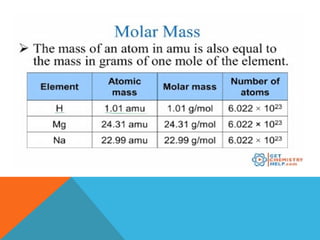
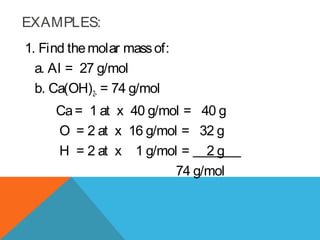
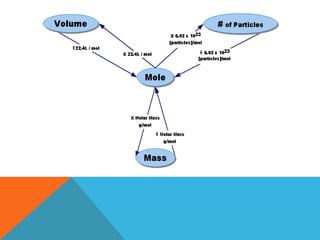
The document explains stoichiometry, focusing on the relationships between elements and compounds during chemical transformations, utilizing the mole concept and skills to balance equations. It covers calculations involving molar mass, Avogadro's number, and percentage composition of compounds, providing exercises for practice. Key definitions and examples demonstrate how to solve problems related to mass relations, molecular weight, and composition of various substances.