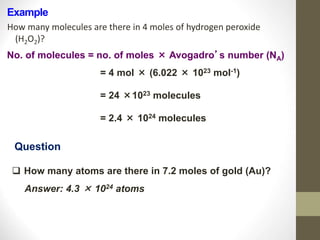
The document discusses the mole concept in chemistry. Some key points:
- A mole is the amount of substance containing Avogadro's number (6.022x1023) of elementary entities like atoms, molecules, formula units.
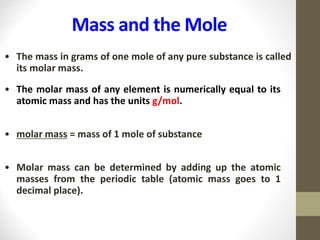
- One mole of any substance has a mass in grams equal to its formula/molar mass. For example, 1 mole of iron (Fe) has a mass of 55.85 g.
- The mole can be used to convert between the number of particles/formula units and the mass of a substance using molar mass and the definition of 1 mole.
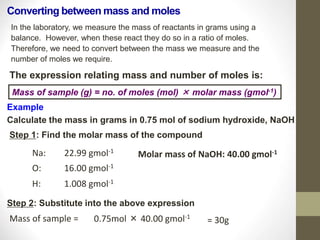
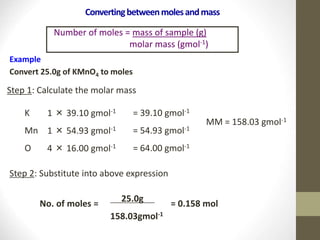
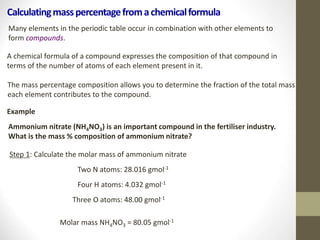
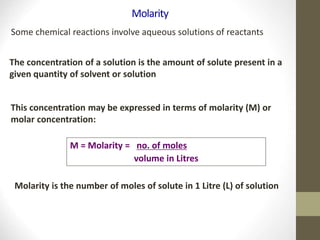
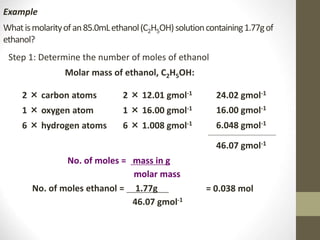
- Common calculations include determining moles from mass or vice versa using molar mass, as well as






![Q. Find the molar mass of CH4.
= 1C + 4H
= 1 x (12.0) + 4 x (1.0)
= 16.0 g/mol
Q. Find the molar mass of Mg(OH)2.
=1Mg + 2O + 2H
=1 x (24.3) + 2 x (16.00) + 2 x (1.0)
=58.3 g/mol
Q. Find the molar mass of MgSO4•7H2O.
=1Mg + 1S + 4O + 7(H2O)
=1 x (24.3) + 1 x (32.1) + 4 x (16.00) + 7 x [2 x (1.0) + 16.00]
=246.4 g/mol](https://image.slidesharecdn.com/chapter1-moleconcept-221026190256-fa2db04e/85/Chapter-1-mole-concept-ppt-11-320.jpg)










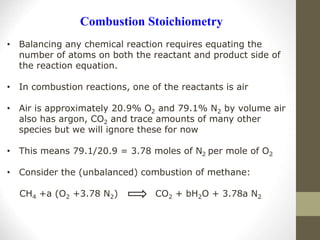

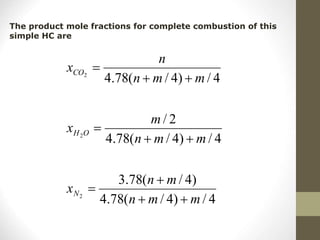
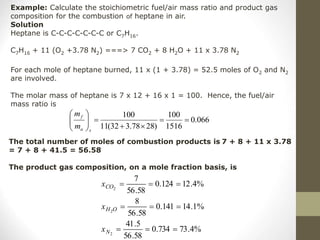
![• In general, a simple HC fuel with the composition CnHm will
undergo complete oxidation to form CO2 and H2O.
CnHm + (n +m/4)(O2+3.78 N2) n CO2 +m/2 H2O + 3.78 (n+m/4)N2
• For each mole of fuel burned, (n + m/4) x (1 + 3.78) = 4.78
x (n + m/4) moles of O2 and N2 are involved, and moles of
combustion products are generated is given as:
Moles of combustion product generated
=n + m/2 + 3.78n + 3.78m/4
=4.78n + m/4 + m/4 + 3.78m/4
=4.78(n + m/4) + m/4
The molar stoichiometric fuel-to-air ratio is
1 / [4.78 x (n + m/4) ]](https://image.slidesharecdn.com/chapter1-moleconcept-221026190256-fa2db04e/85/Chapter-1-mole-concept-ppt-30-320.jpg)