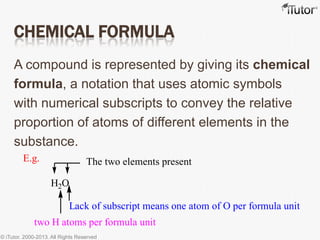
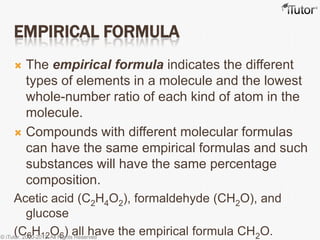
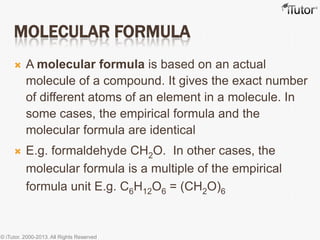
This document discusses different types of chemical formulas used to represent compounds:
- A chemical compound is made of two or more elements that are combined in fixed ratios.
- The chemical formula uses symbols of the elements and subscripts to show the relative proportions.
- The empirical formula shows the lowest whole number ratio of atoms in a compound, while the molecular formula gives the exact number of each atom.
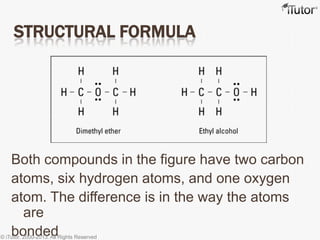
- The structural formula depicts the specific bonding arrangement of the atoms in a compound.