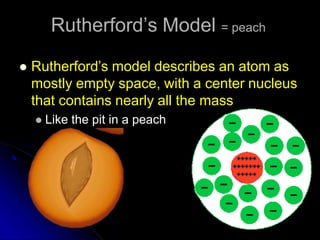
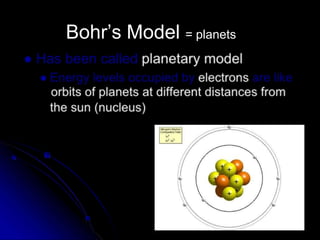
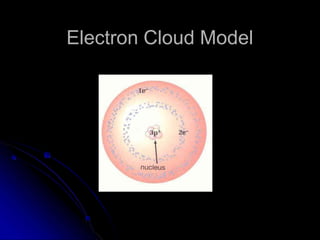
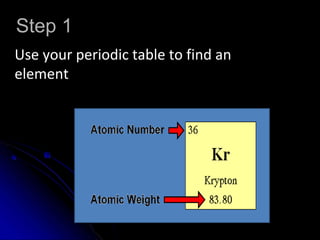
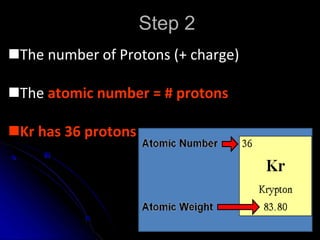
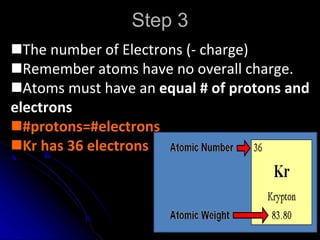
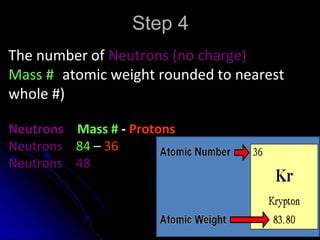
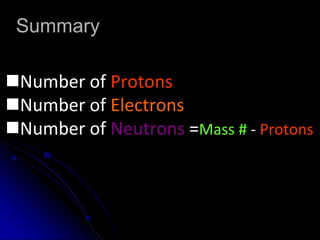
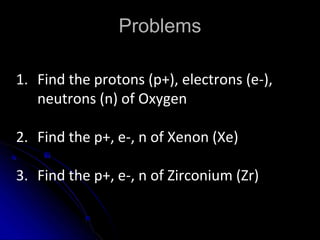
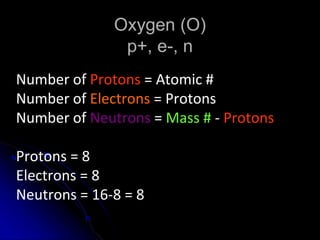
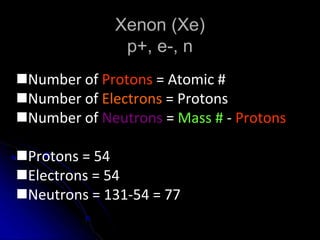
The atomic-molecular theory of matter states that all matter is composed of small, fast-moving particles called atoms that can join together to form molecules. This theory has developed over thousands of years through the works of scientists like Democritus, Dalton, Thomson, Rutherford, Bohr, Chadwick, and others who proposed and tested successive atomic models. The current model depicts atoms made up of a small, positively charged nucleus surrounded by a negative electron cloud.