1) The document discusses atoms, elements, compounds, and mixtures. It aims to explain what an atom is, differentiate between elements, compounds and mixtures, and give examples of each.
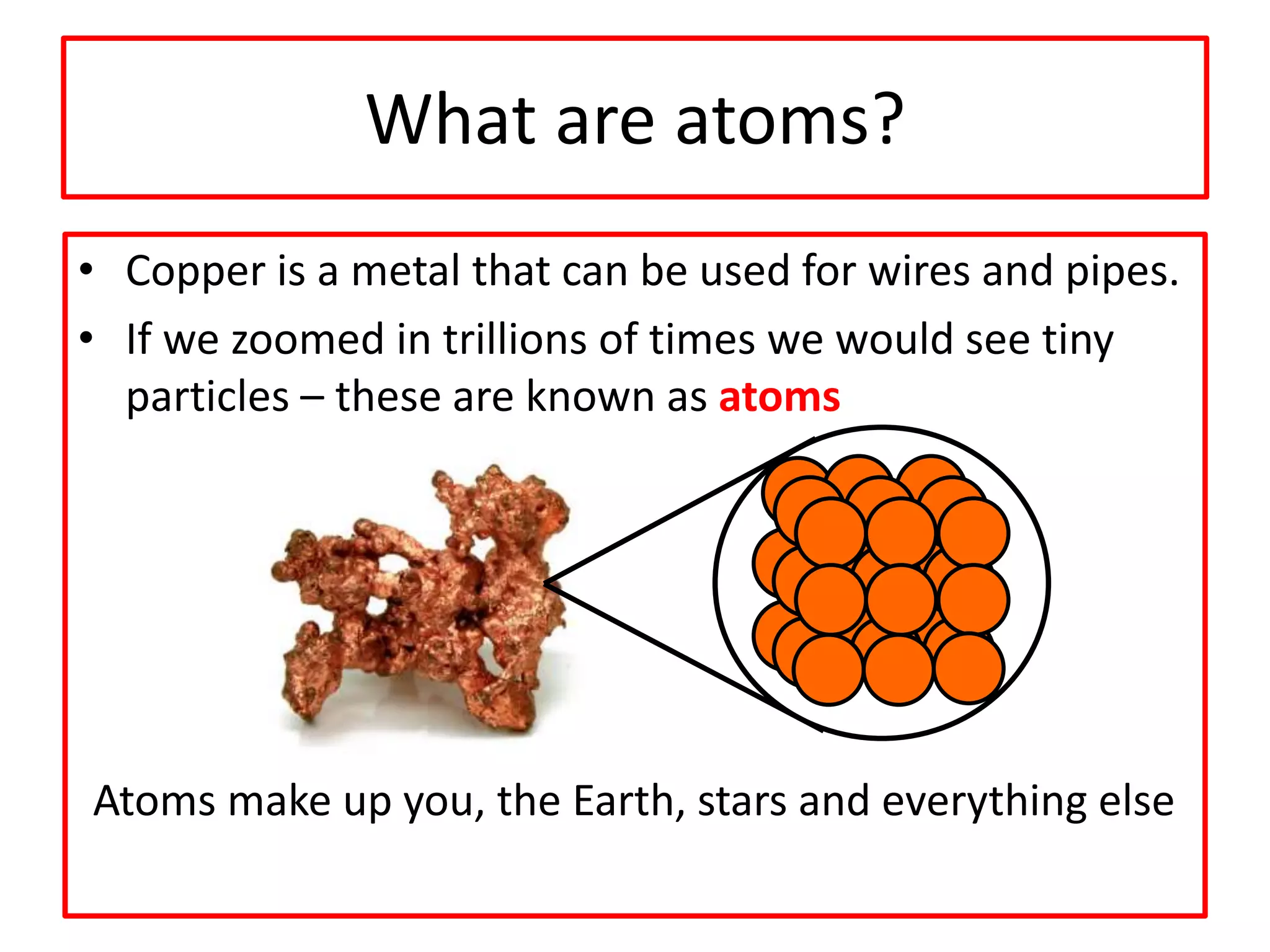
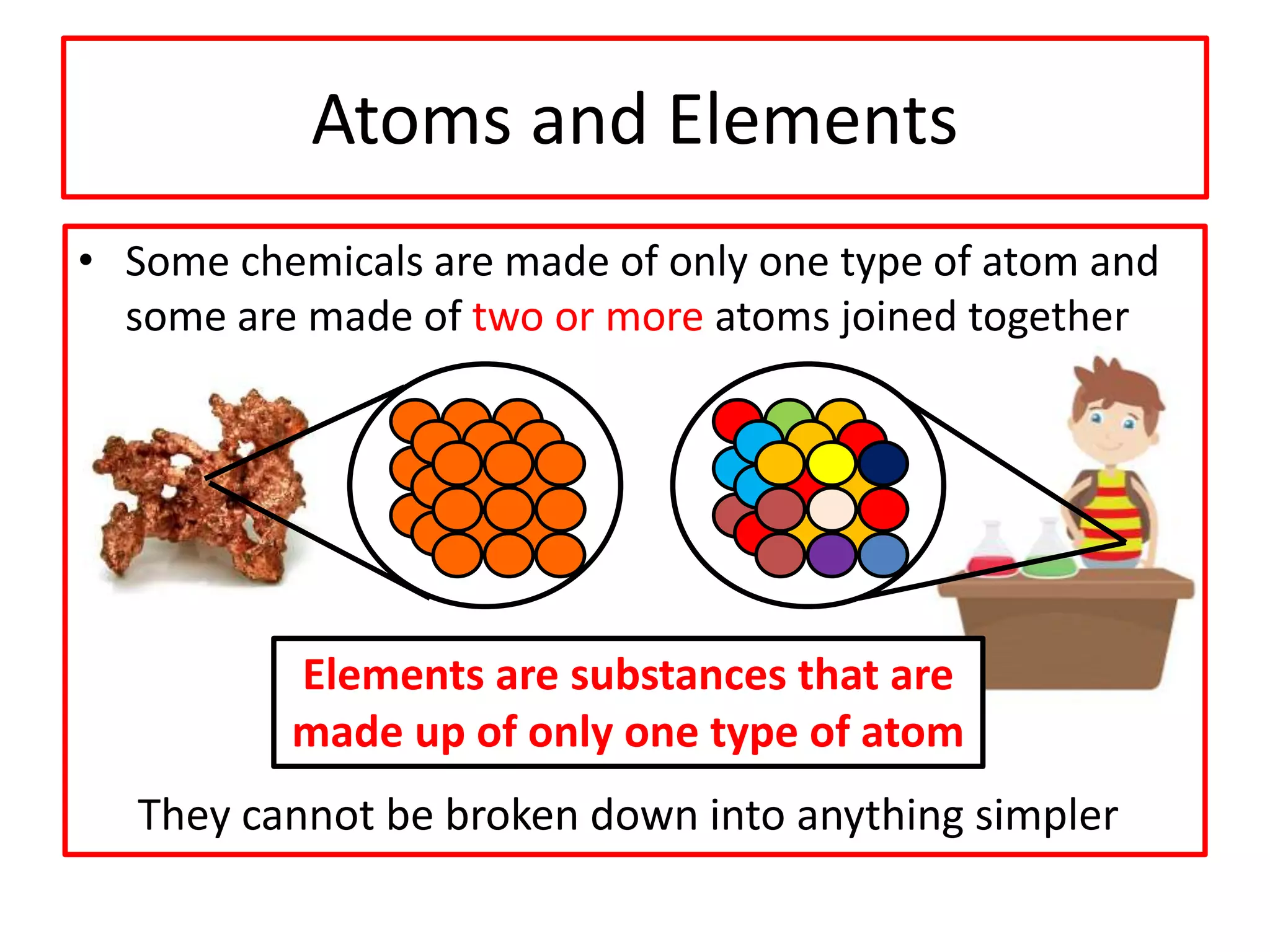
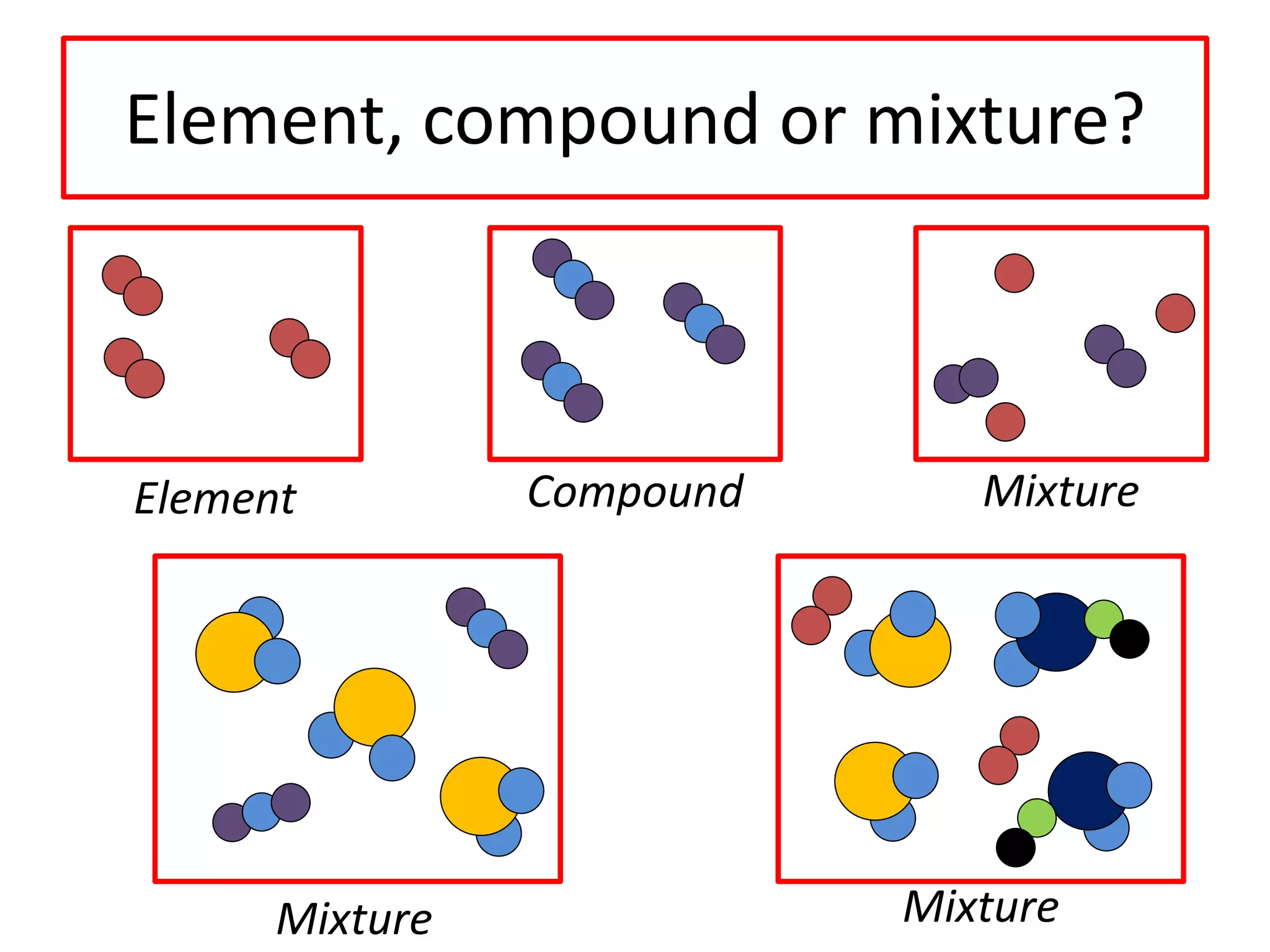
2) Atoms are the basic building blocks of all matter and are very small. Elements are substances made of only one type of atom that cannot be broken down further.
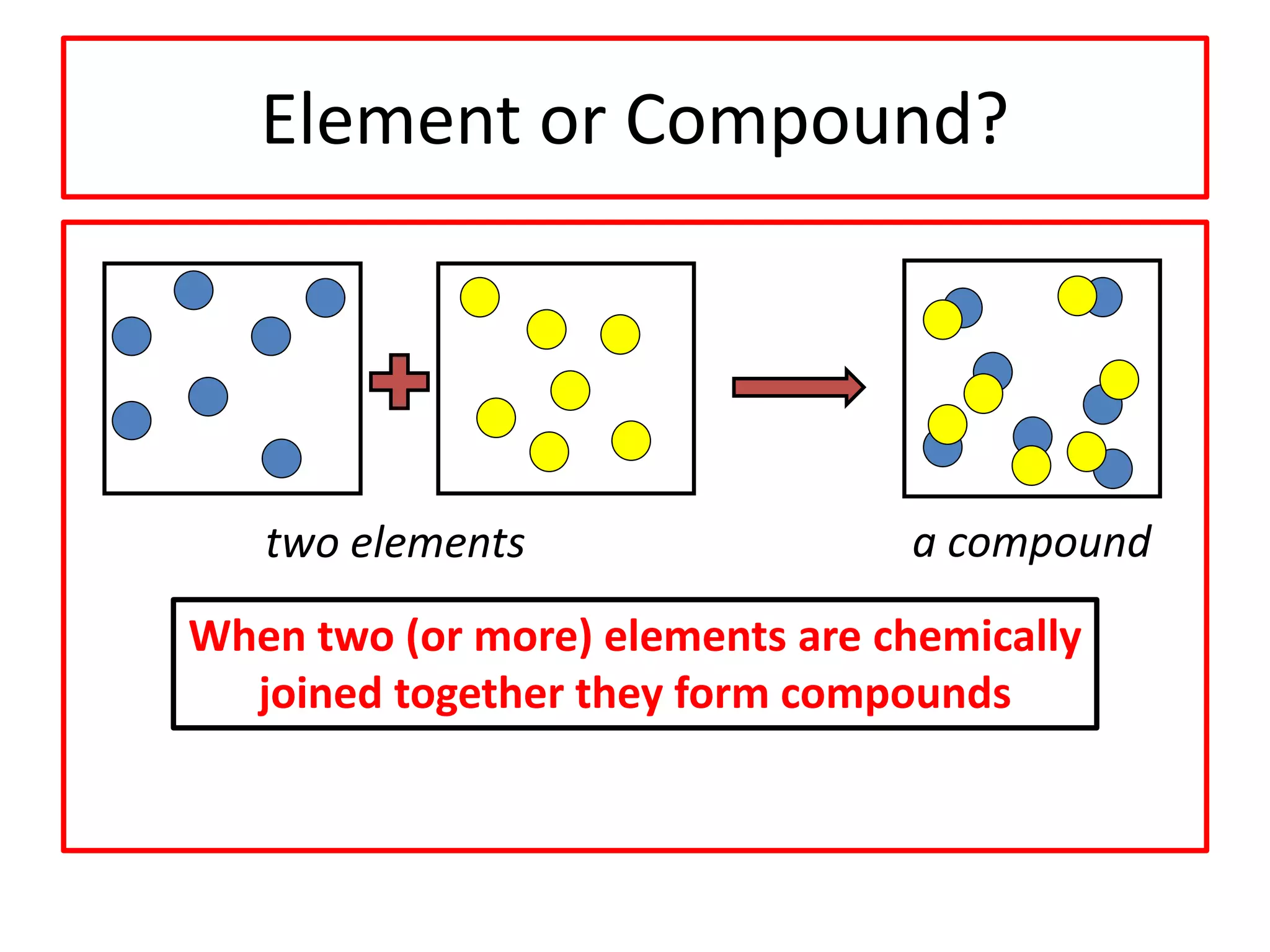
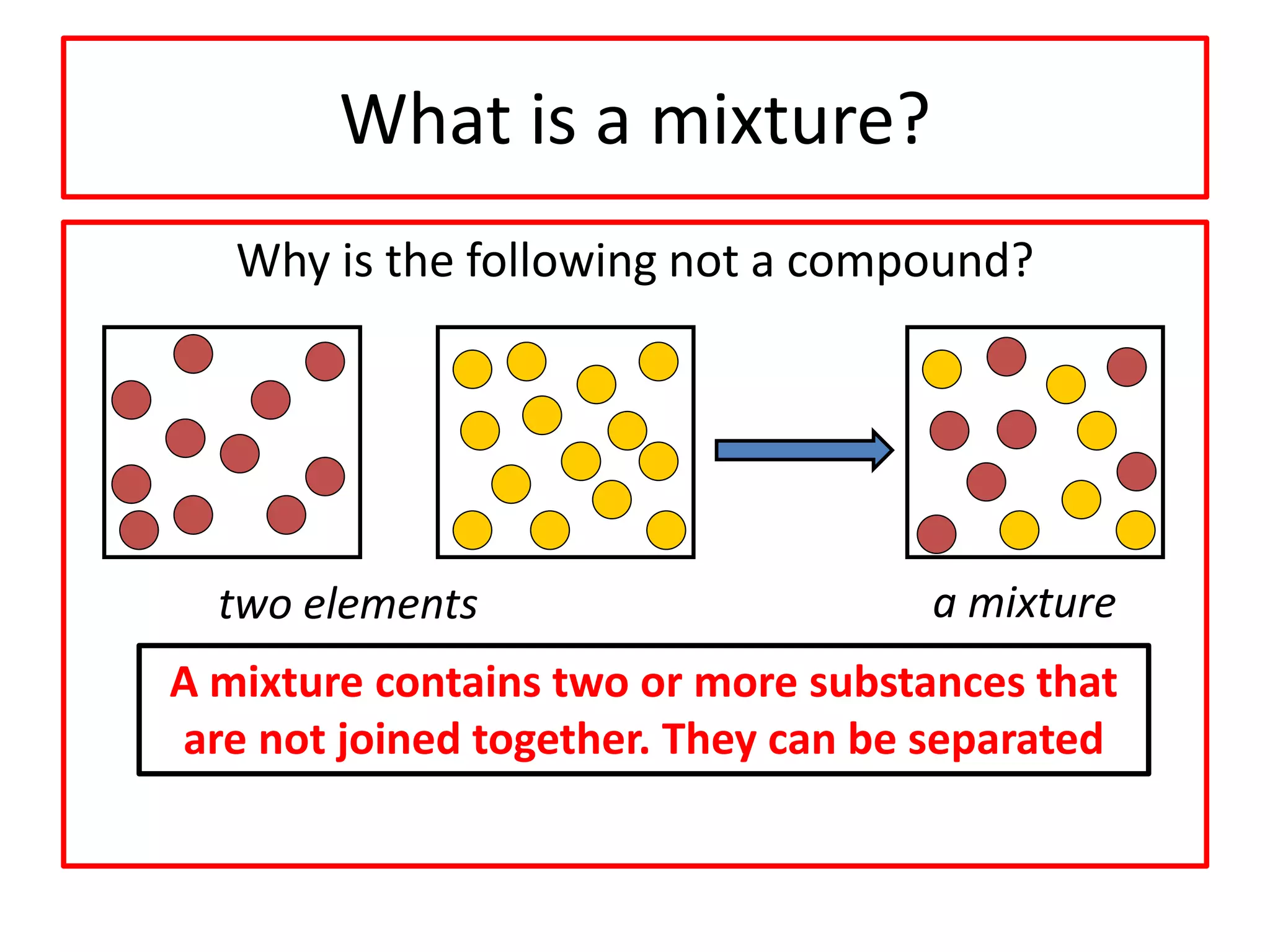
3) Compounds are formed when two or more elements are chemically bonded together and have different properties than the original elements. Mixtures contain two or more substances that are not chemically bonded and can be separated.