This document provides an overview of the structure of an atom. It discusses:
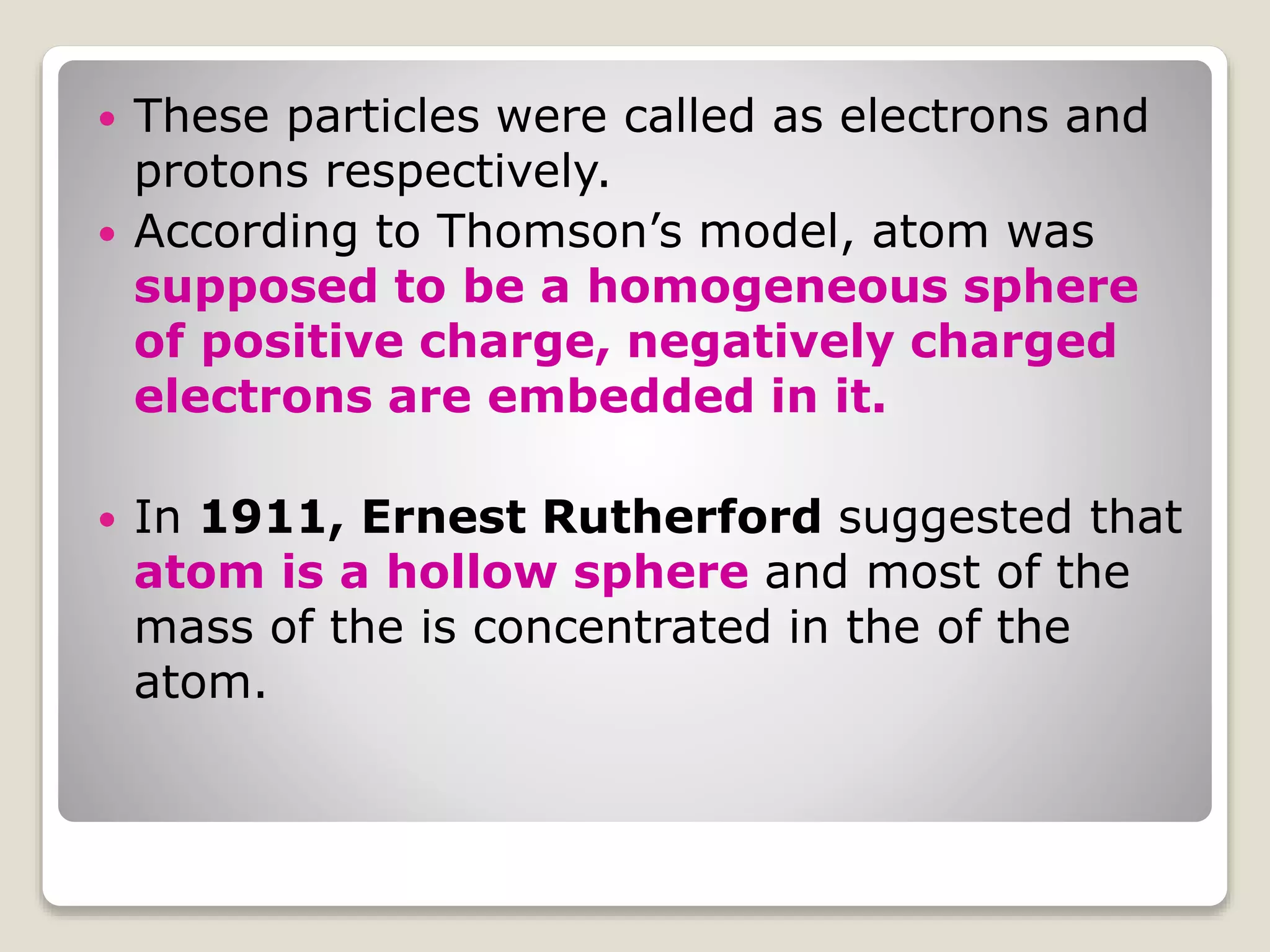
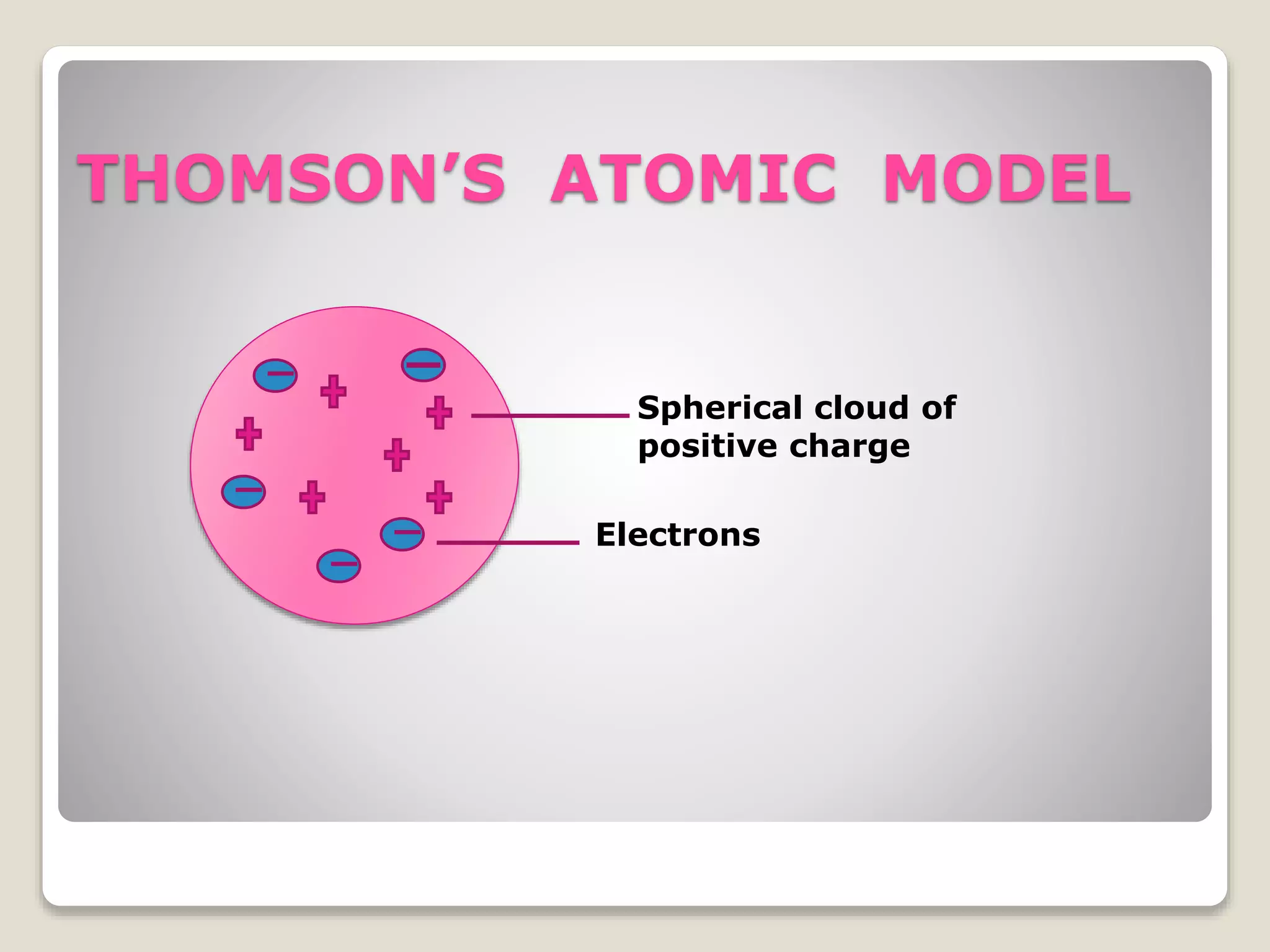
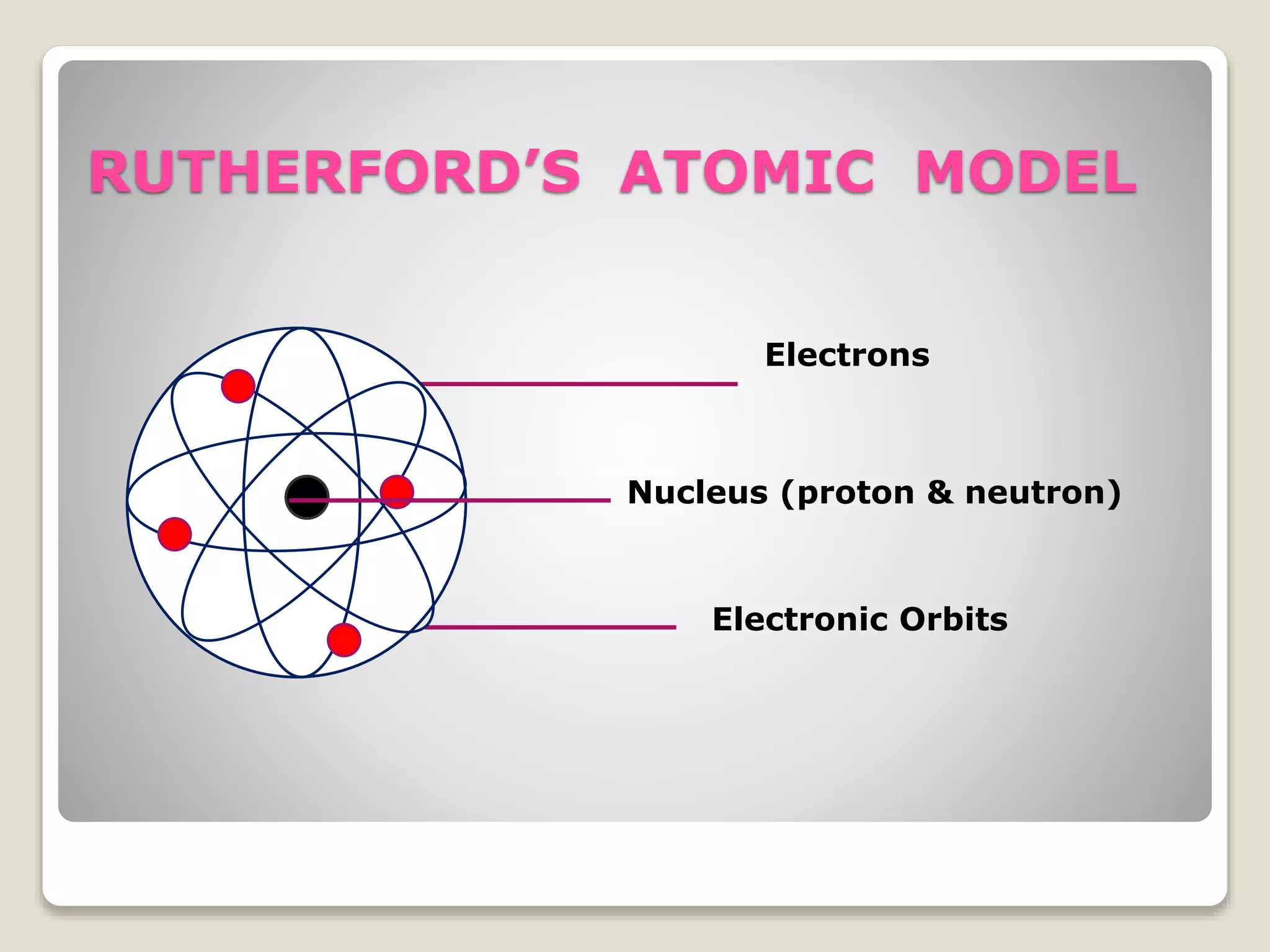
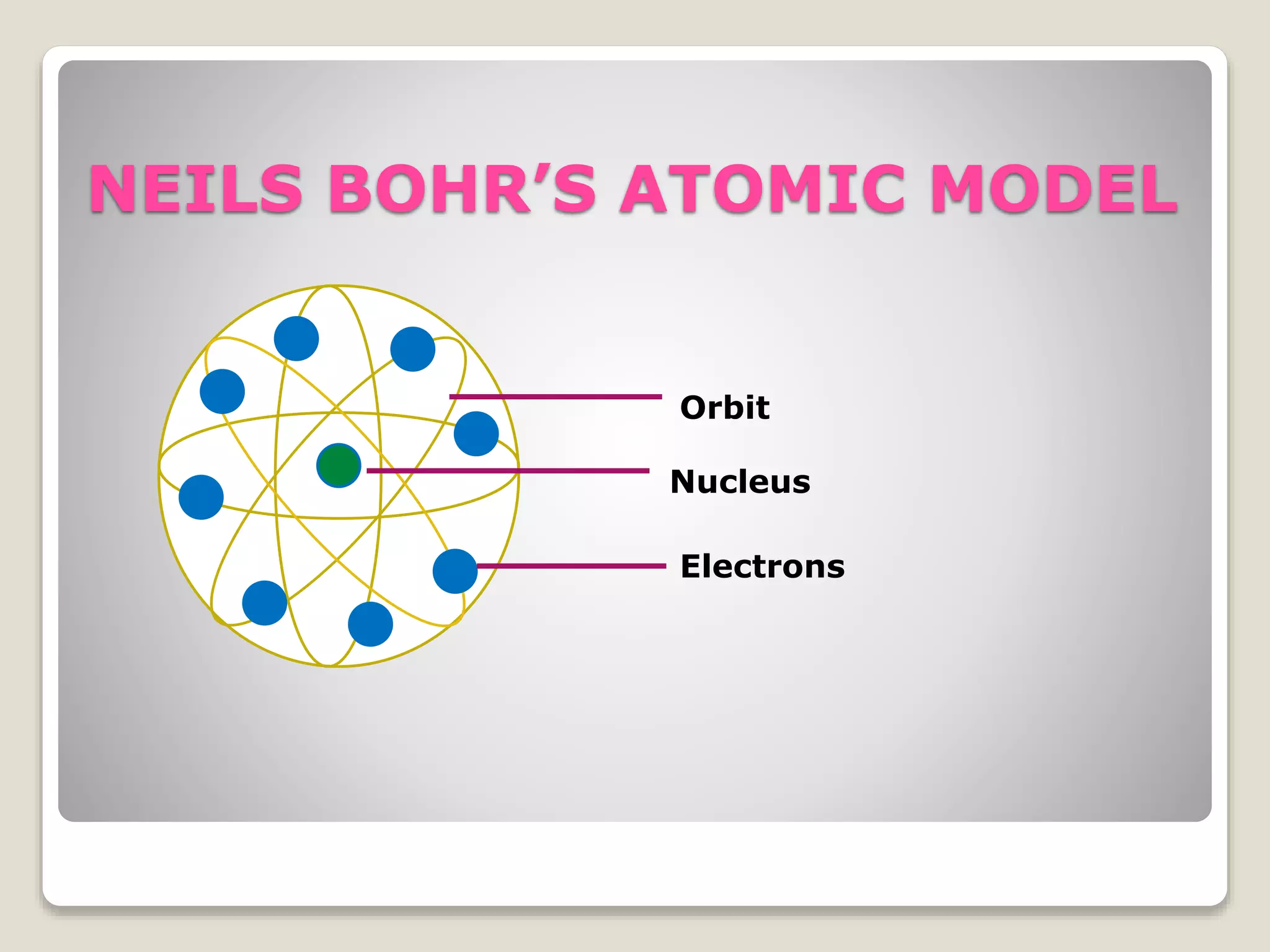
1) Early atomic models including Thomson's model of a uniform positive charge with embedded electrons, and Rutherford's model with a small, dense nucleus and orbiting electrons.
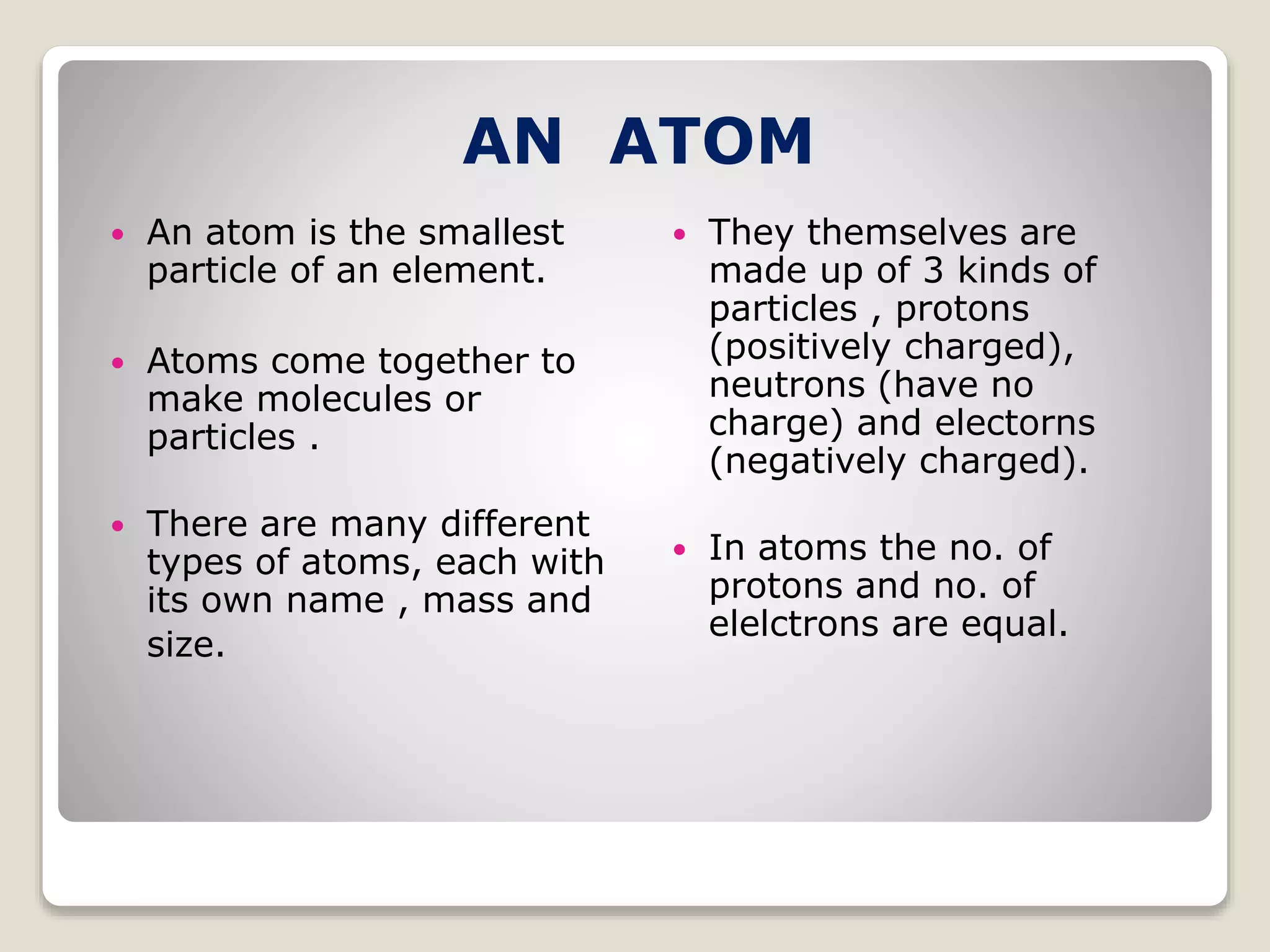
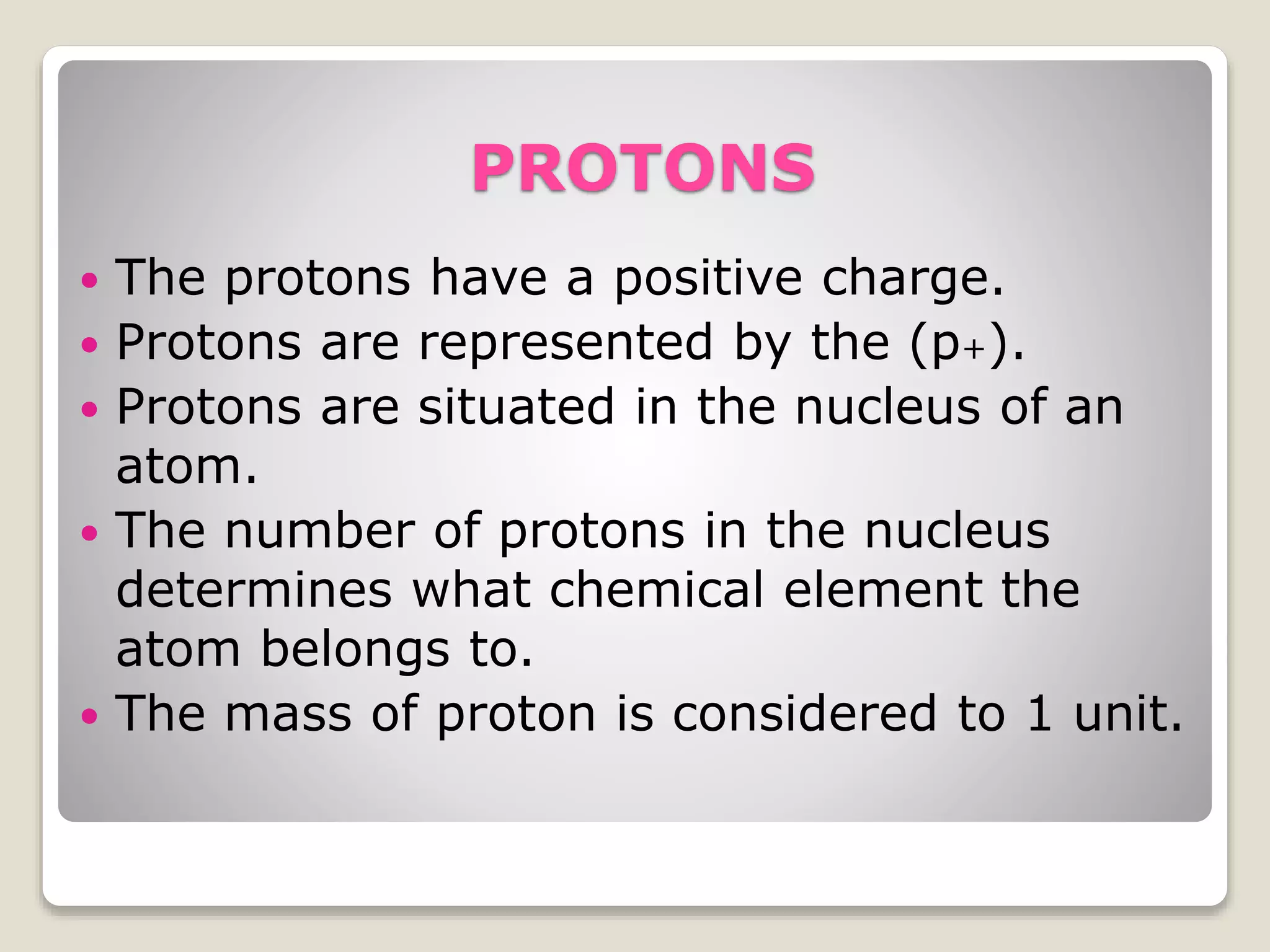
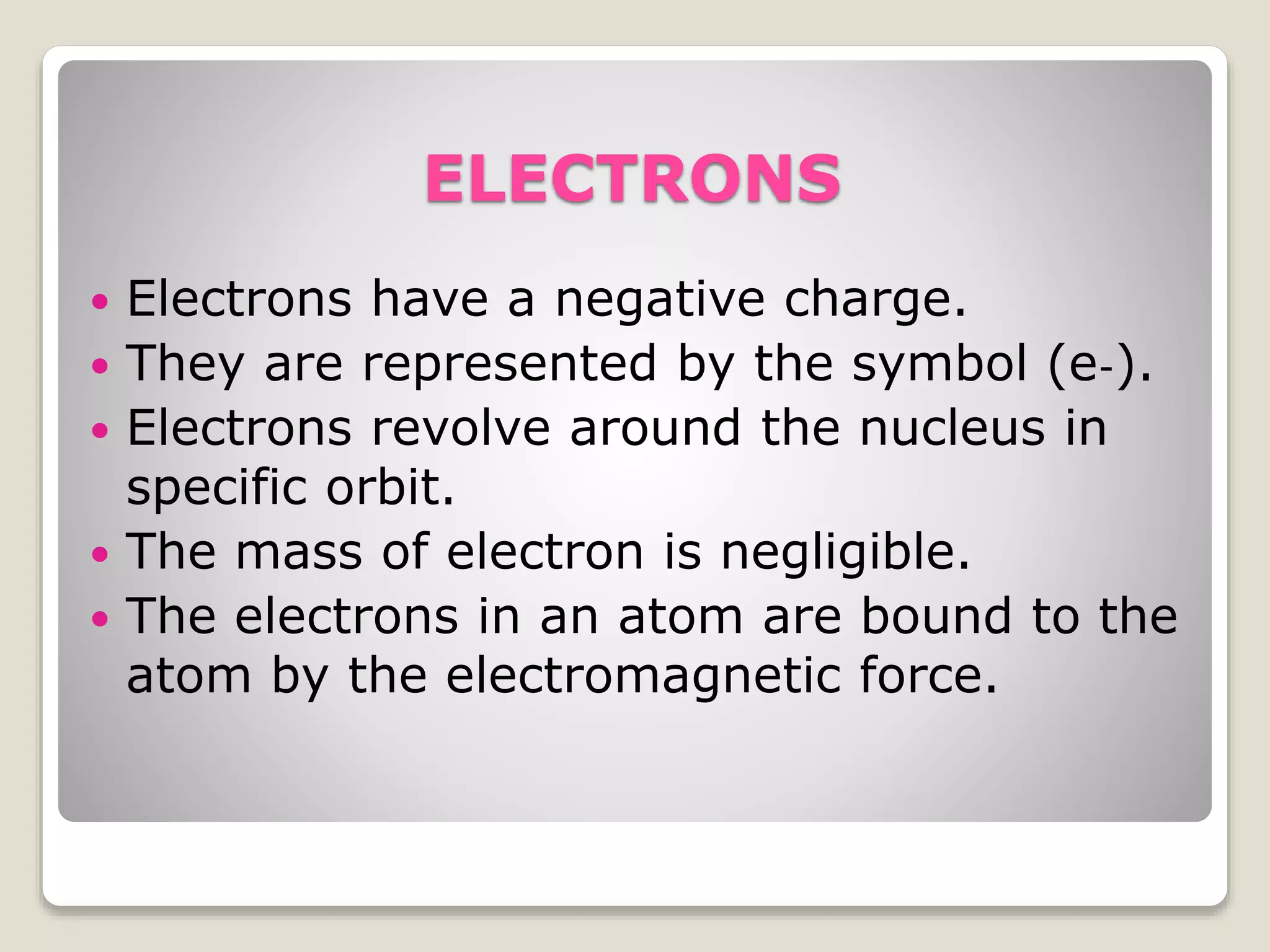
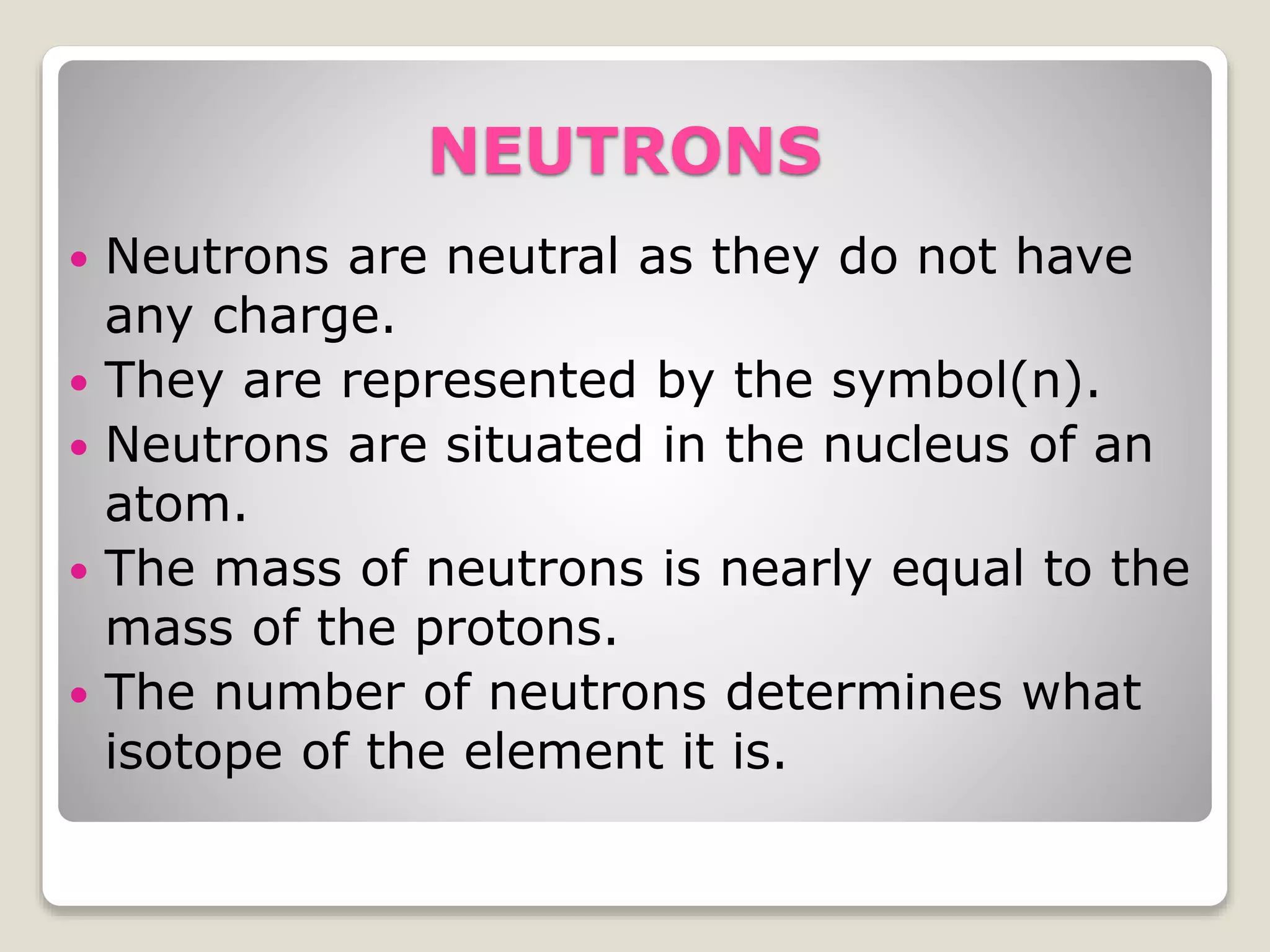
2) The three main subatomic particles - protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge.
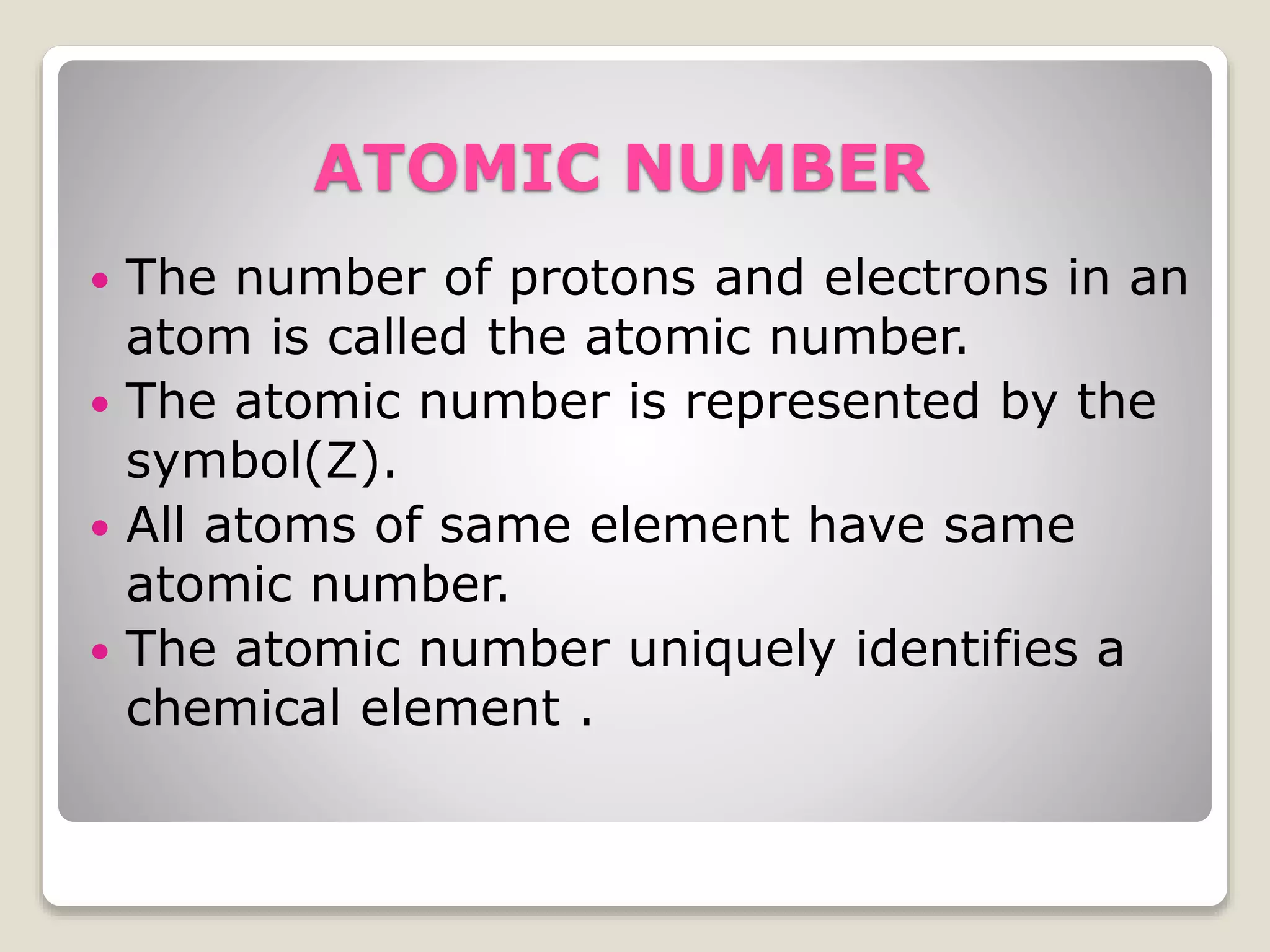
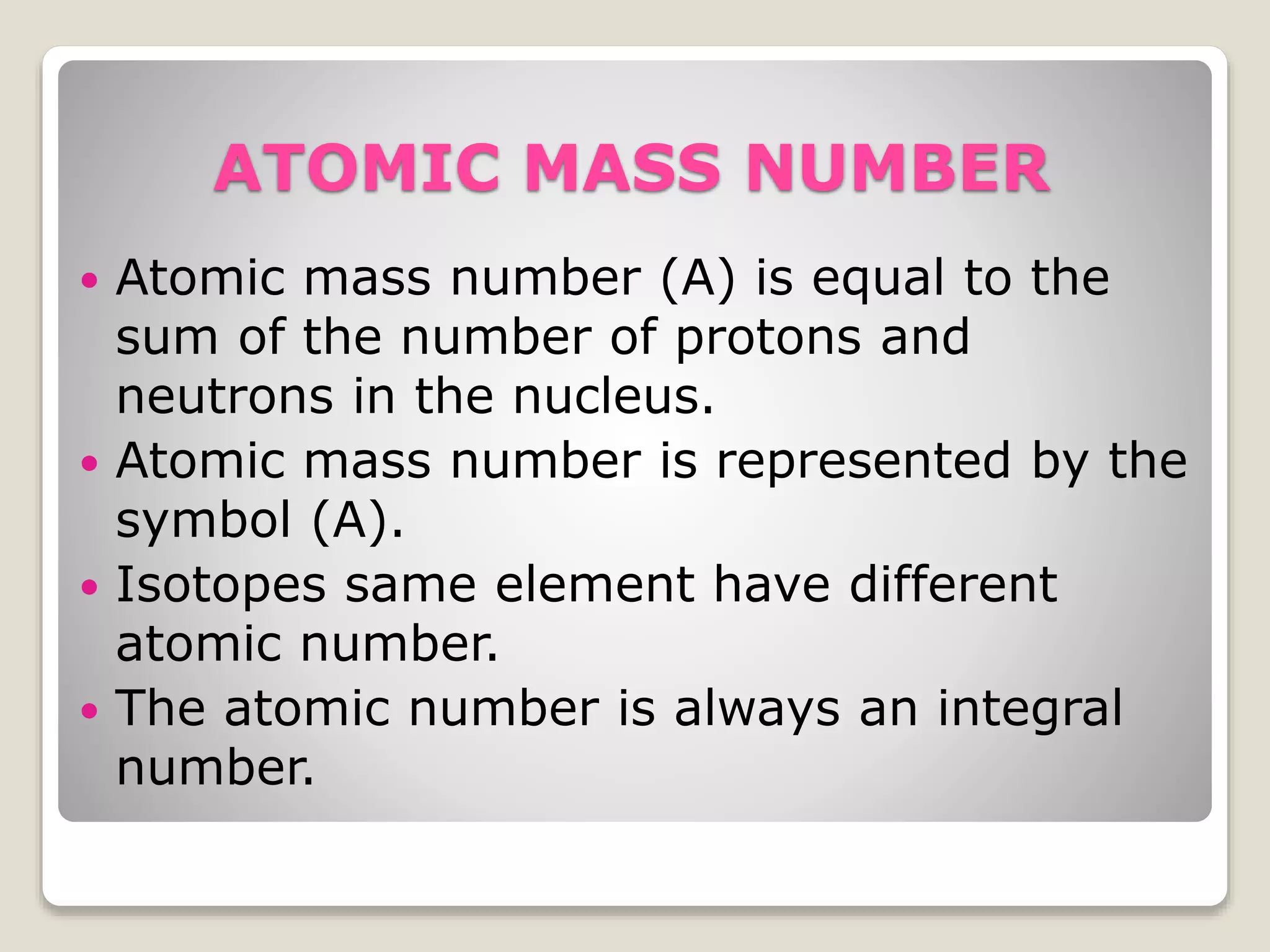
3) Key atomic properties including atomic number, the number of protons; mass number, the sum of protons and neutrons; and atomic weight, the relative weight of an atom compared to hydrogen.
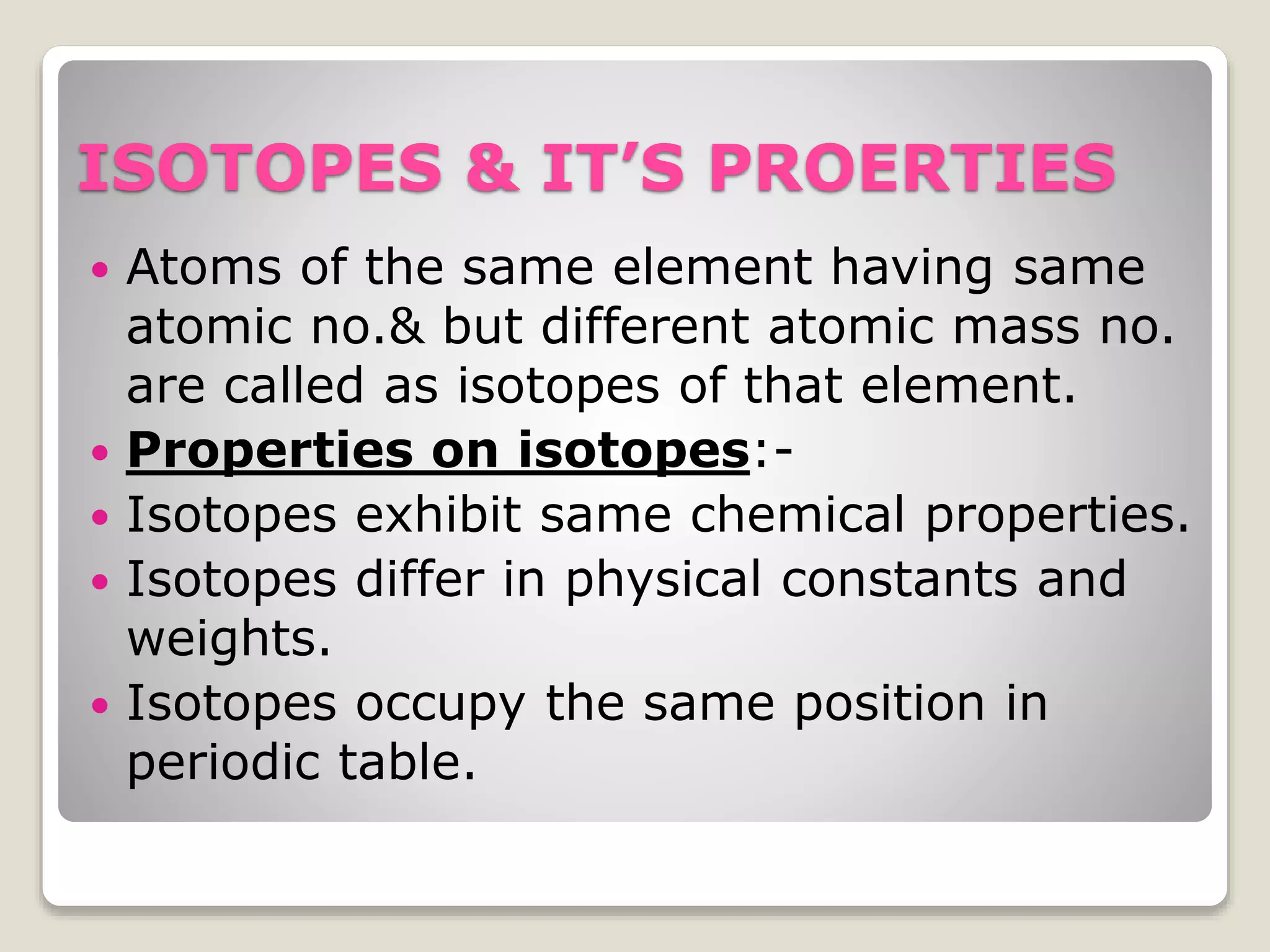
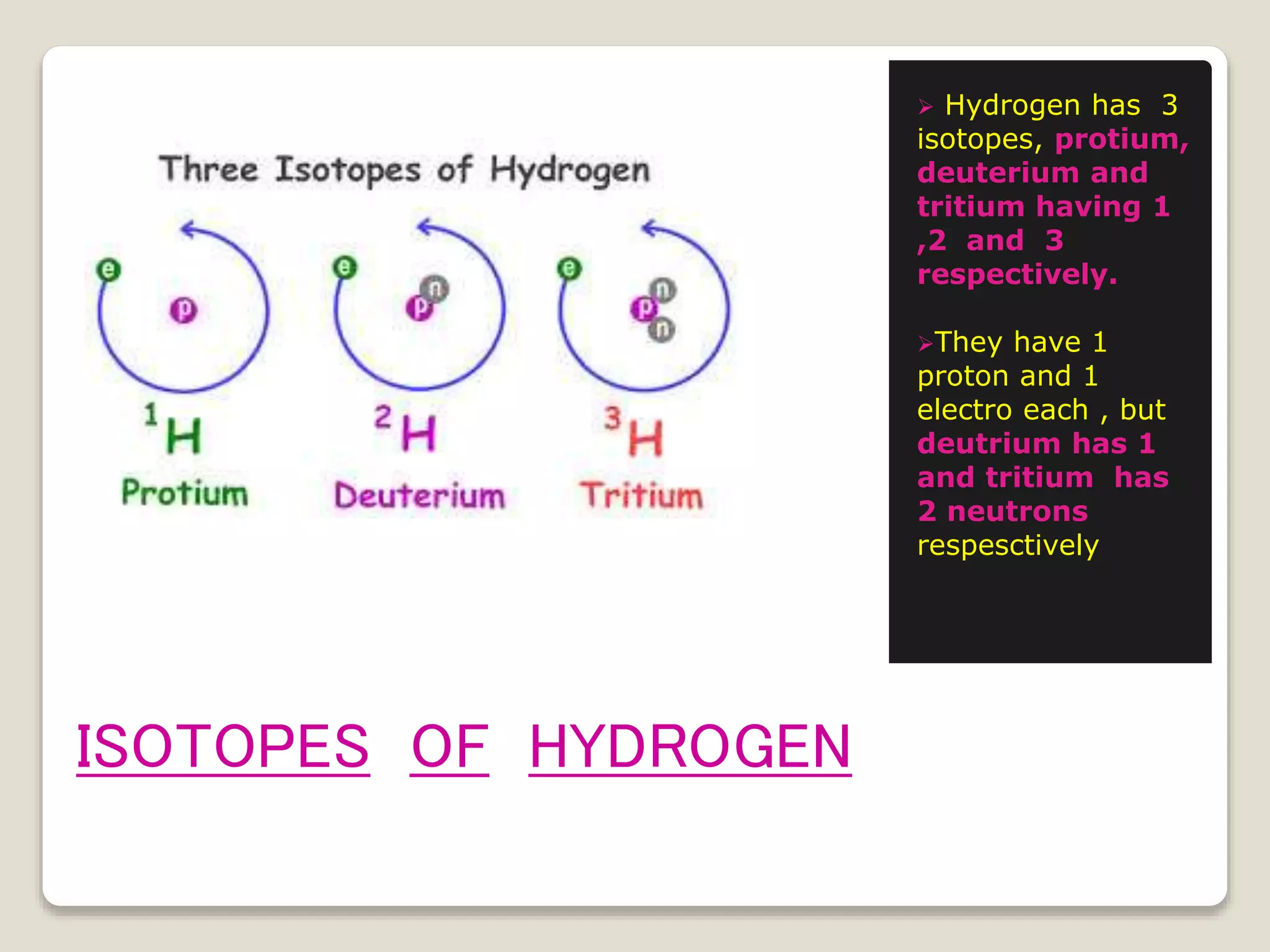
4) Isotopes, atoms of the same element with the