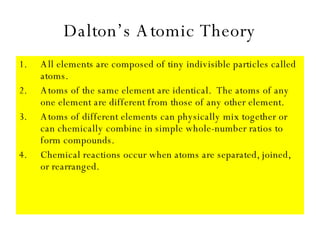
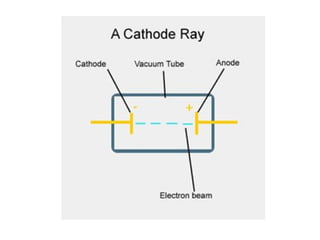
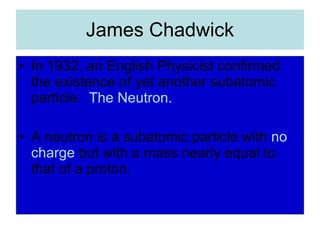
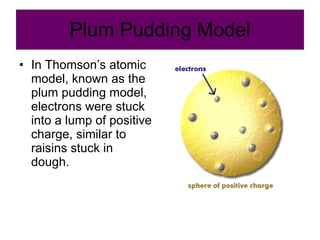
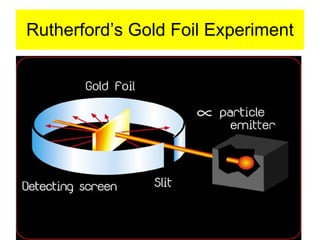
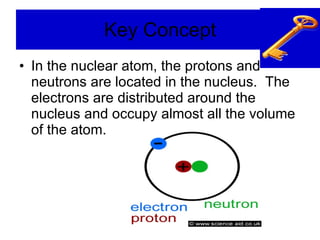
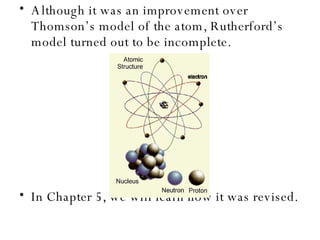
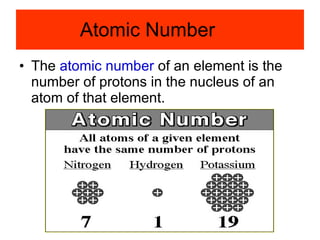
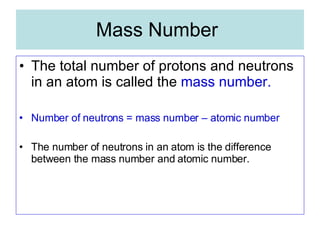
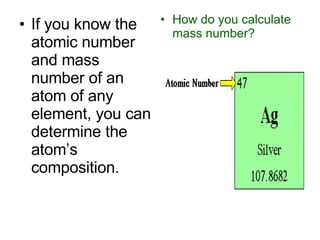
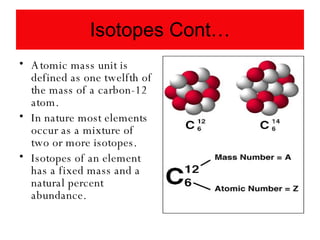
Chapter 4 explores atomic structure, defining the atom as the smallest particle of an element that retains its identity, with historical perspectives from Democritus to Dalton's atomic theory, which highlights that atoms are indivisible and can combine in whole-number ratios. It discusses the discovery of subatomic particles—electrons, protons, and neutrons—and introduces the nuclear atom model proposed by Rutherford, emphasizing the nucleus's concentration of positive charge and mass. The chapter also covers distinctions among elements via atomic number, mass number, and isotopes, culminating in a preview of the periodic table's organization.