The document provides an overview of atomic structure and models of the atom over time. It discusses:
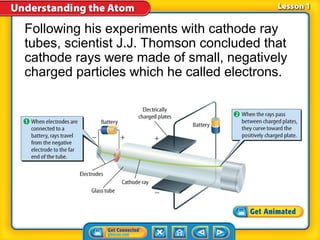
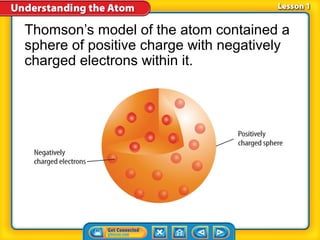
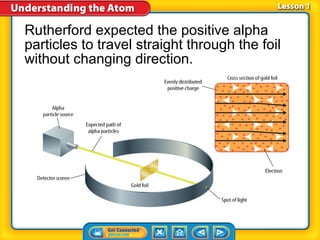
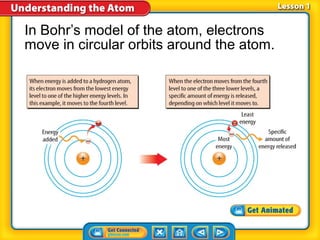
1) Early atomic models including Thomson's model of a positively charged sphere with electrons inside and Rutherford's discovery of the nucleus at the atom's center.
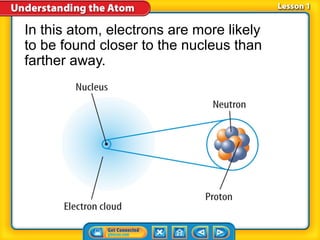
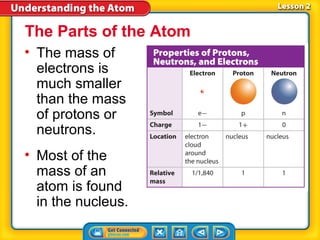
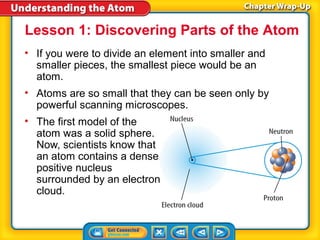
2) Modern atomic structure with a dense nucleus surrounded by an electron cloud, and that protons and neutrons are made of quarks.
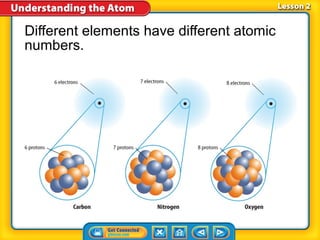
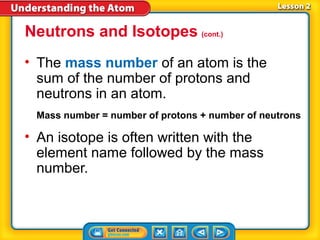
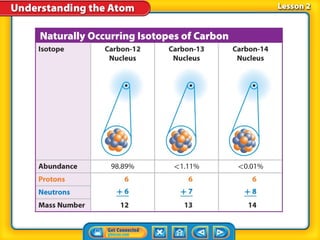
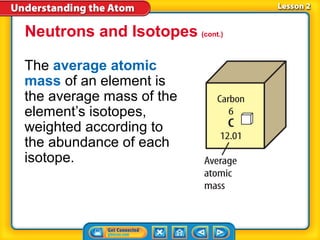
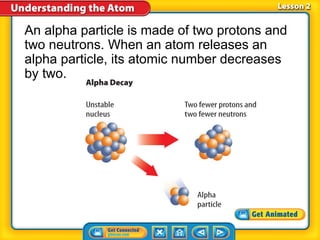
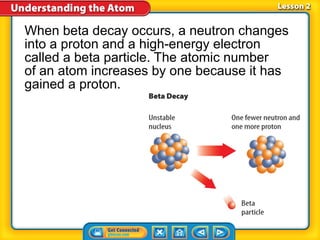
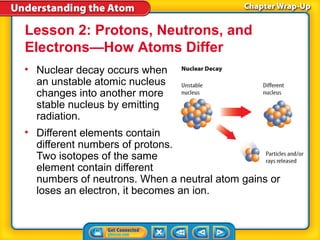
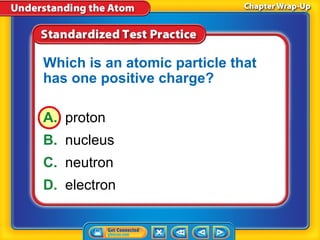
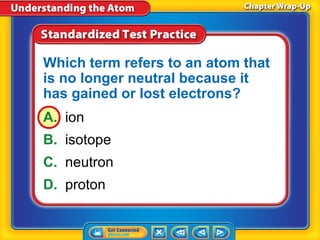
3) Differences between atoms including atomic number, isotopes having different neutron numbers, and ions forming when atoms gain or lose electrons.