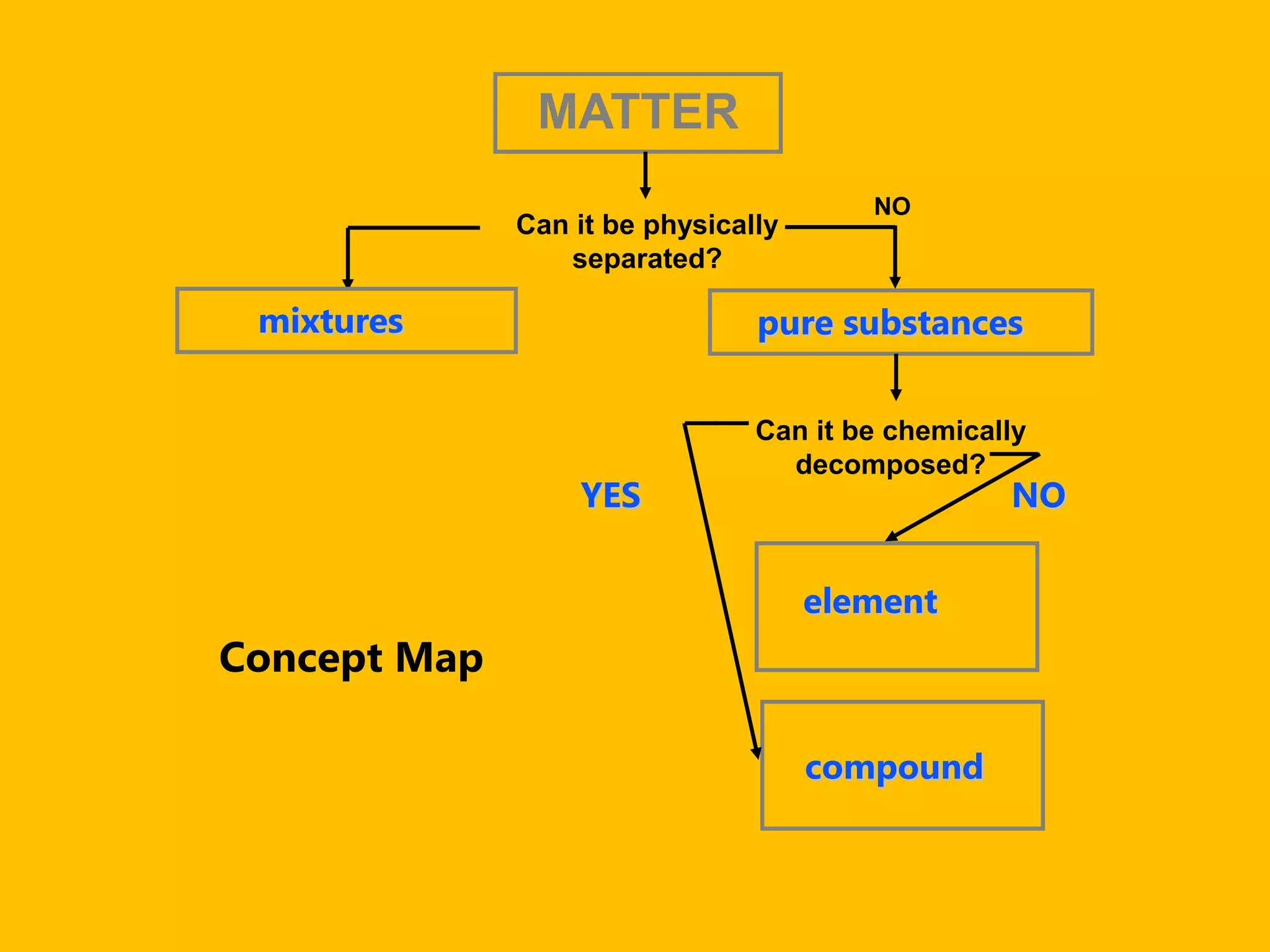
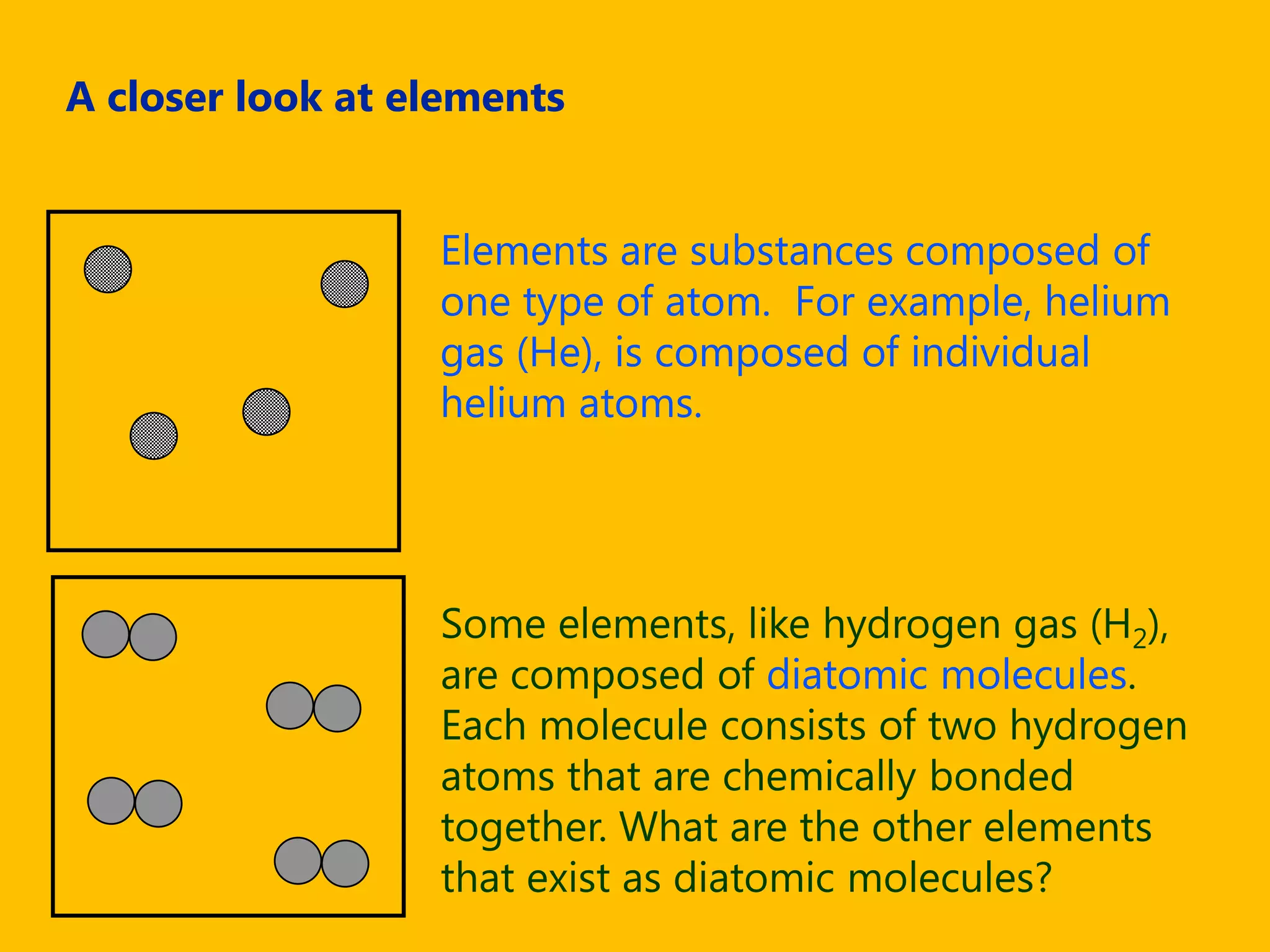
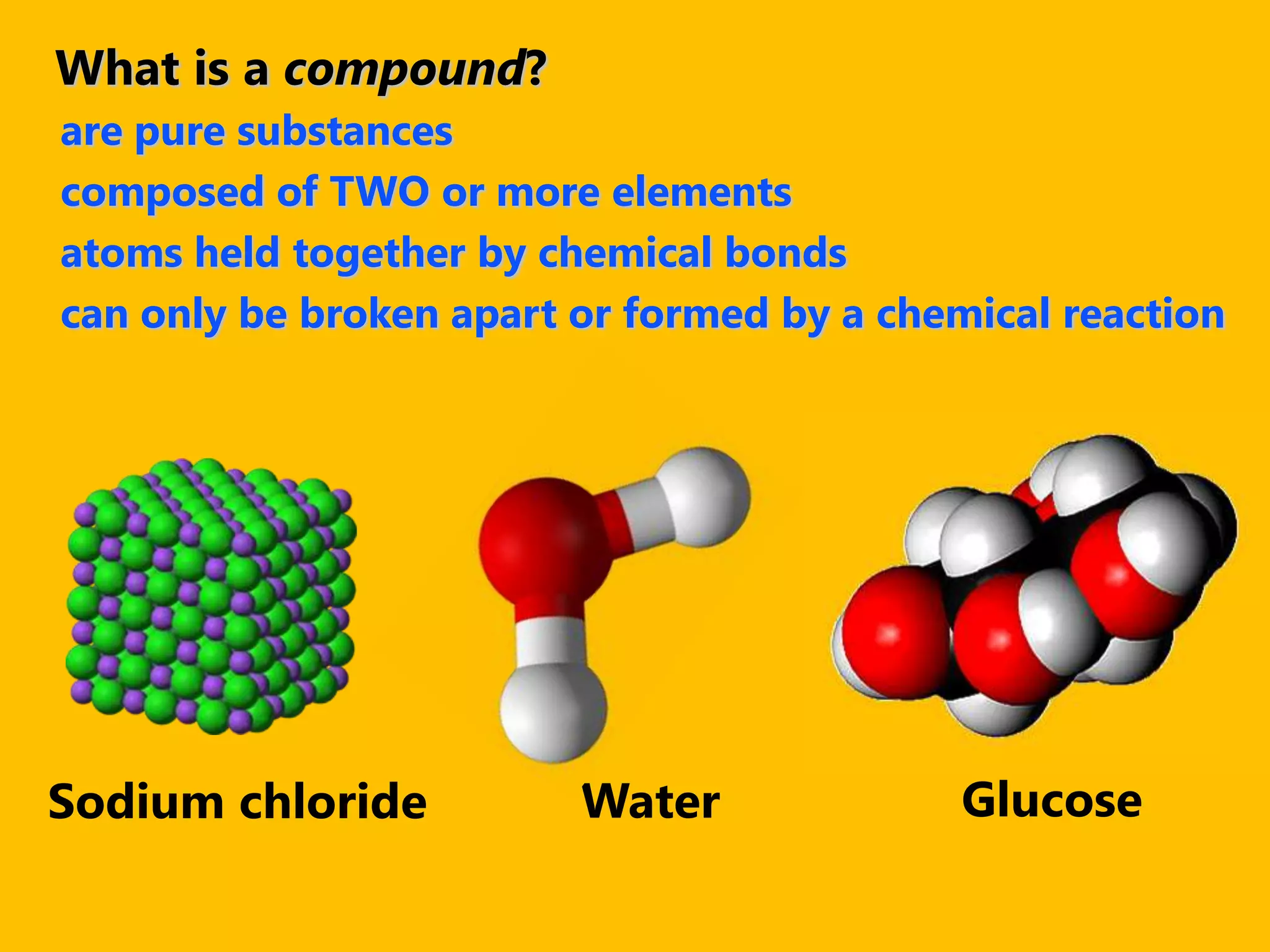
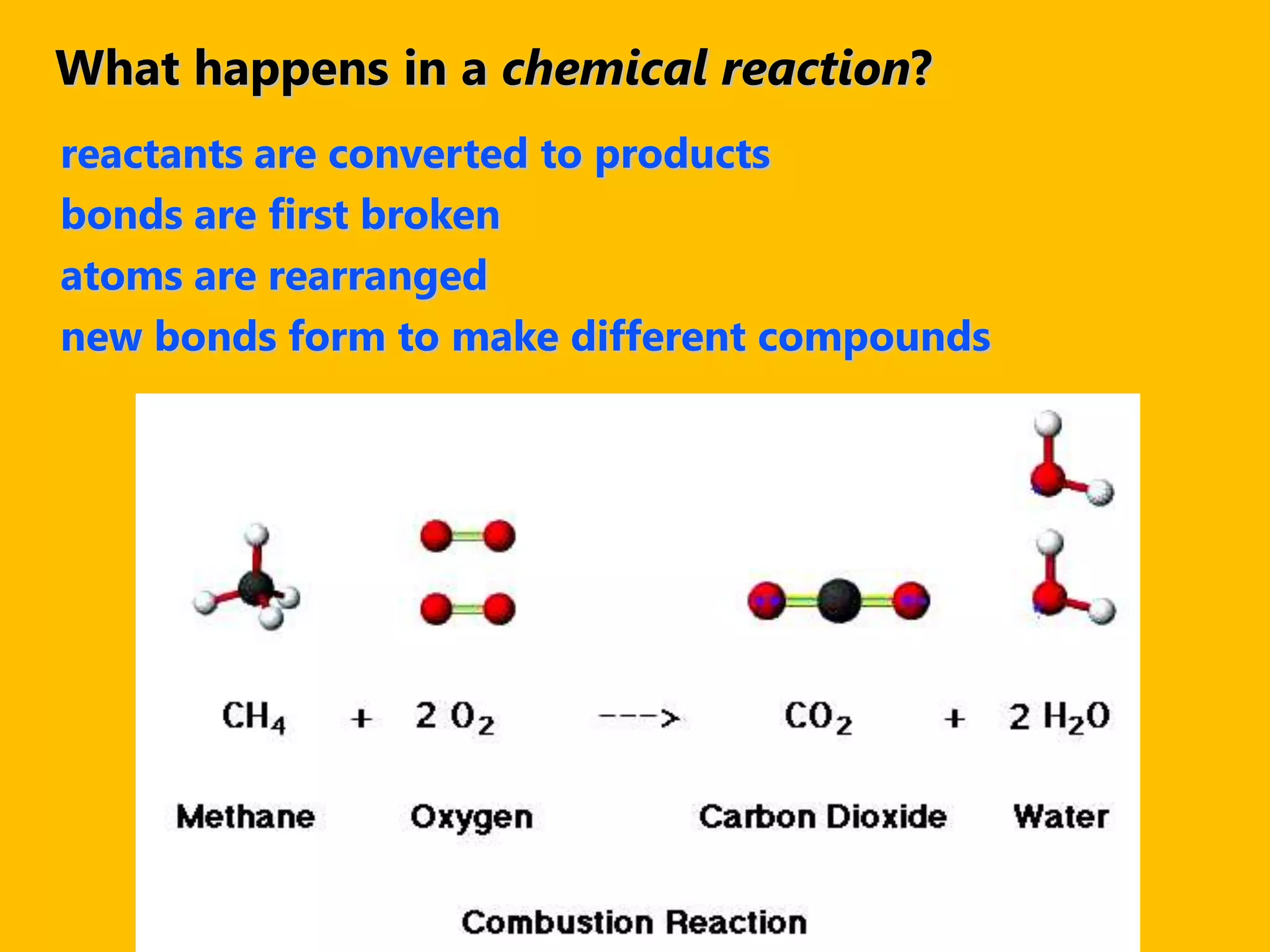
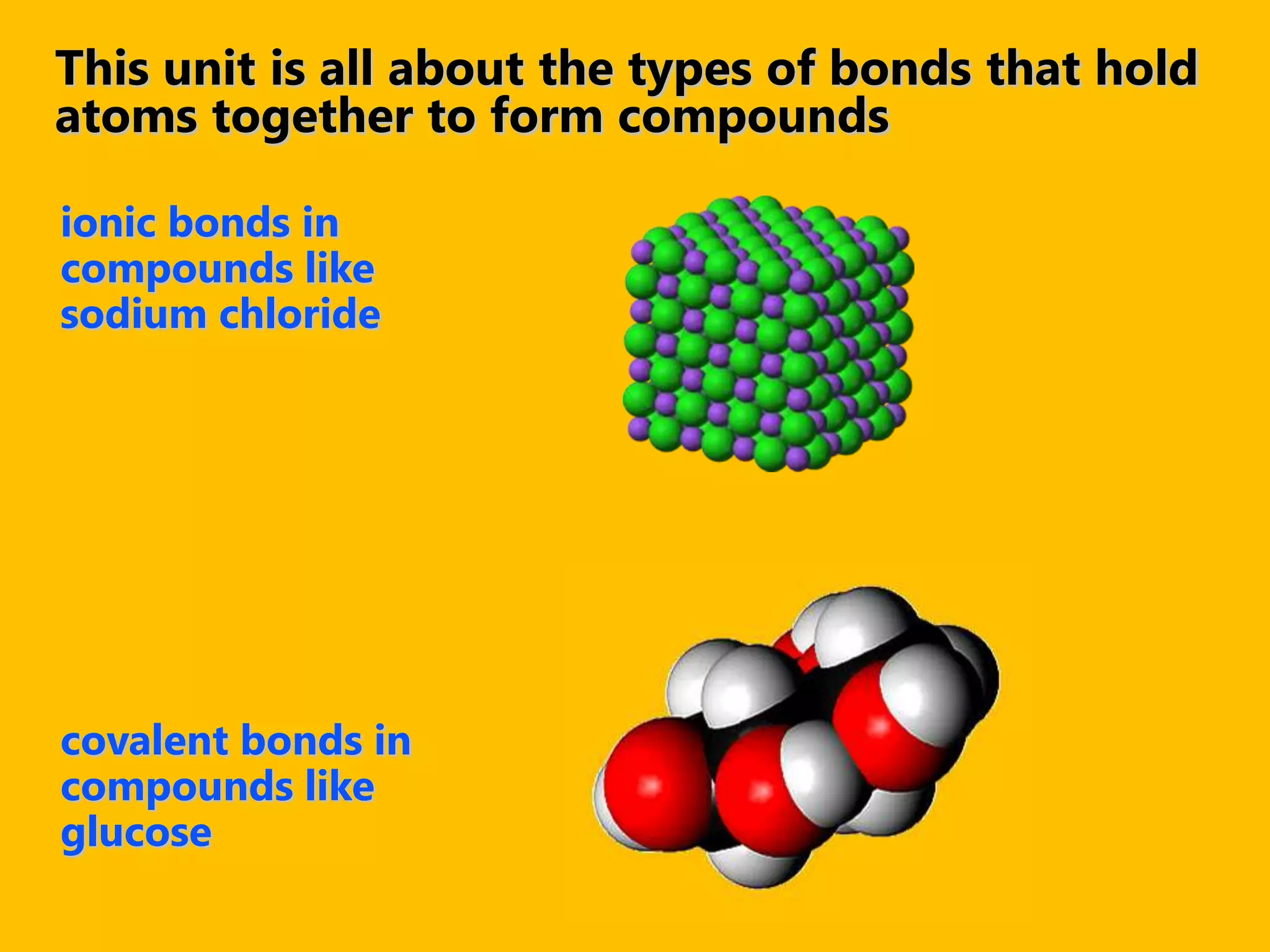
A compound is a pure substance composed of two or more elements chemically bonded together, such as sodium chloride or water. Compounds can only be broken down or formed through a chemical reaction that breaks and rearranges bonds between atoms. Some common compounds found in homes include sodium chloride, water, glucose, and other substances containing ionically or covalently bonded elements.