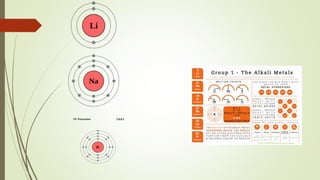
The document explains the concept of matter, atoms, and their structure, detailing the components of an atom including protons, neutrons, and electrons. It discusses the periodic table, developed by Henry Moseley, which organizes elements by atomic number into groups and periods, indicating similarities in chemical properties. Additionally, it categorizes elements into groups such as alkali metals, alkaline earth metals, and halogens based on their reactivity and valence electrons.