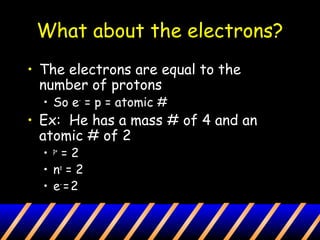
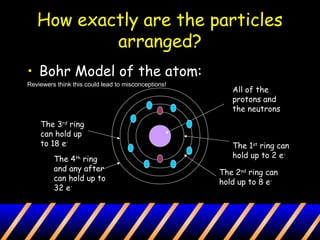
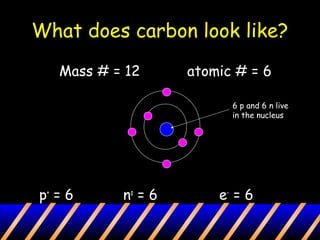
Atoms are composed of a nucleus containing protons and neutrons surrounded by an electron cloud. The nucleus contains positively charged protons and neutral neutrons. Negatively charged electrons reside outside the nucleus in the electron cloud. The number of protons defines the identity of an element and is equal to its atomic number. The total number of protons and neutrons is the mass number. The number of electrons equals the number of protons to maintain electroneutrality. Models such as the Bohr model depict electron arrangement in shells surrounding the nucleus.