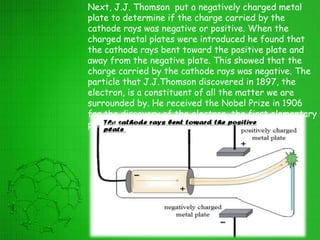
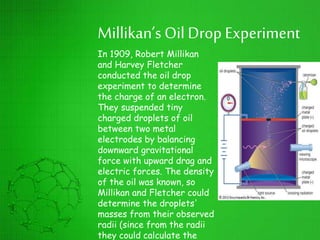
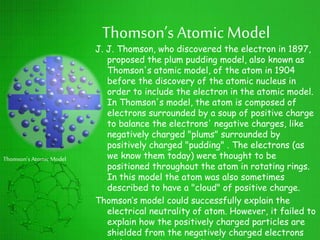
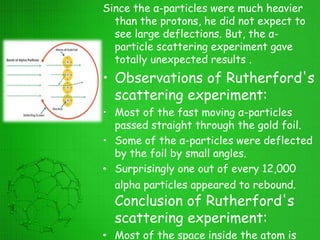
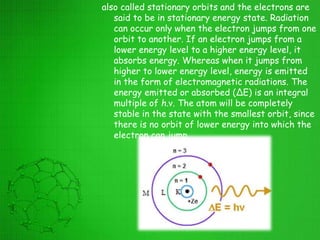
The document discusses the history of the development of atomic structure models from Thomson's plum pudding model to Rutherford's nuclear model. Key events include J.J. Thomson's discovery of the electron, Millikan's oil drop experiment determining the charge of an electron, discovery of the proton through canal ray experiments, Rutherford's alpha particle scattering experiment revealing the dense nucleus at the center of the atom, and Rutherford proposing the nuclear model of the atom. The nuclear model represented a major breakthrough but did not fully explain electron stability.