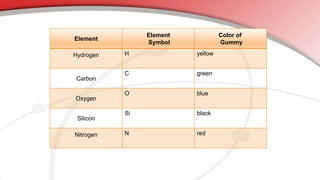
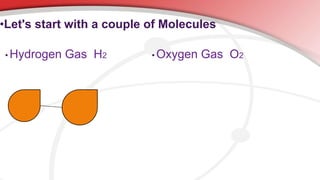
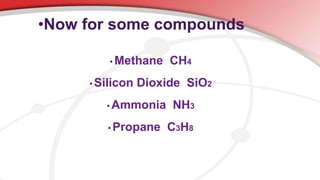
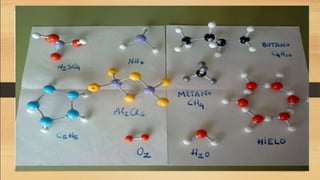
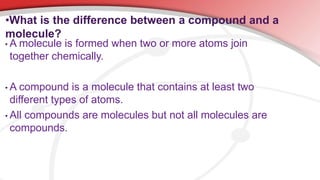
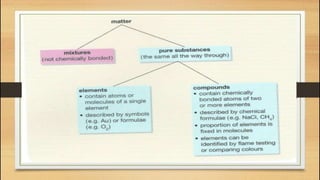
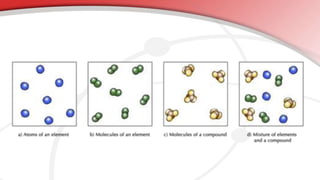
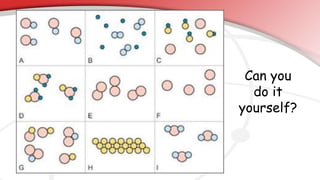
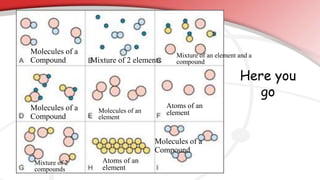
The document defines key chemistry terms including atoms, molecules, elements, compounds, mixtures and solutions. Atoms are the smallest unit of matter that cannot be divided further. Molecules are formed when two or more atoms combine chemically. Matter is made up of elements, compounds, mixtures and solutions. Elements are made of the same type of atom, compounds contain two or more types of atoms bonded together with a specific chemical formula. Mixtures contain substances that are not chemically combined, while solutions occur when a substance dissolves evenly in another.