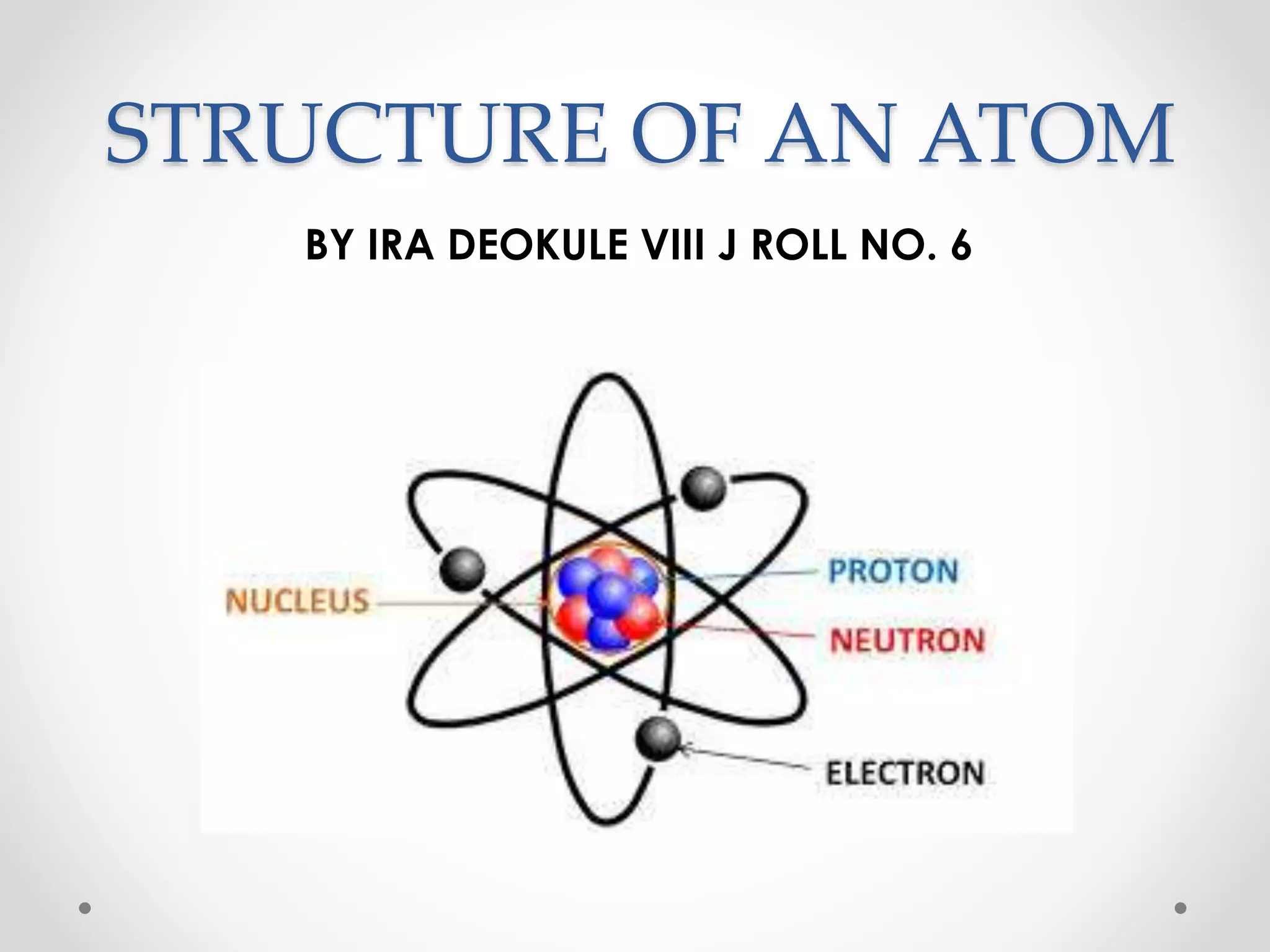
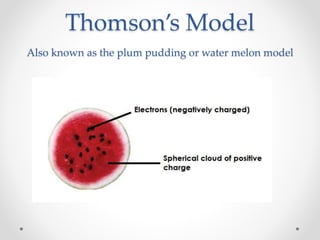
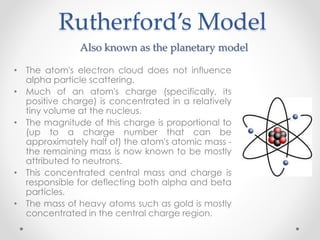
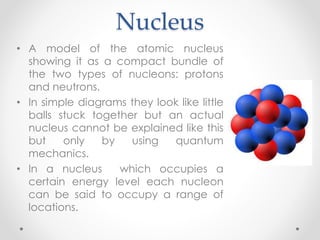
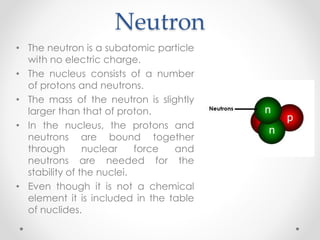
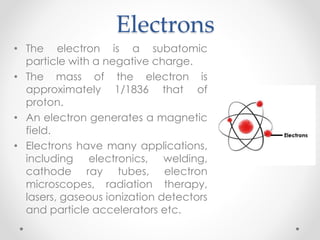
- Dalton's model proposed that all matter is made of tiny particles called atoms, all atoms of the same element are alike but different from other elements, and compounds form from atoms combining in fixed proportions. Thomson's model pictured the atom as a positively charged sphere with electrons embedded within it. Rutherford's model showed that the atom's positive charge and most of its mass are concentrated in a tiny, dense nucleus at the center with electrons orbiting outside. The parts of an atom include the nucleus containing protons and neutrons at its center, surrounded by electrons. Protons have a positive charge while neutrons have no charge. Electrons have a negative charge and much less mass than protons or neutrons.