Embed presentation
Downloaded 186 times
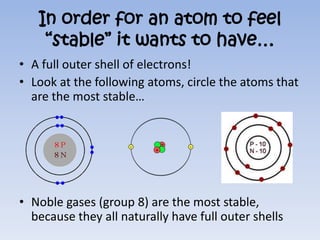

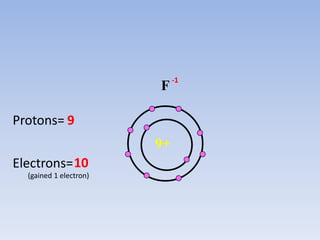
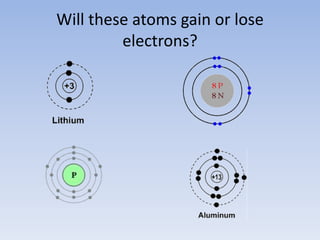

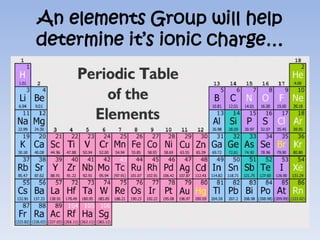
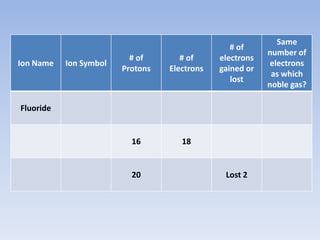
Atoms form ions by gaining or losing electrons to achieve a full outer shell like noble gases. Sodium loses 1 electron to form Na+ with 10 electrons and a positive charge as a cation. Fluorine gains 1 electron to form F- with 10 electrons and a negative charge as an anion. An element's group number helps determine its ionic charge when forming cations or anions to achieve a full outer shell.








