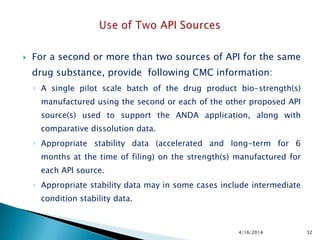
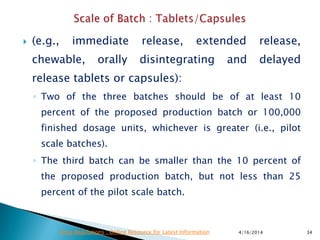
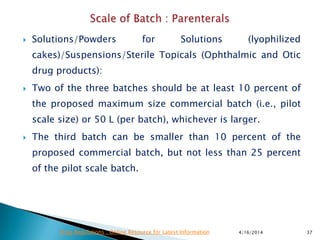
This presentation provides guidance on stability testing requirements for new drug applications (ANDAs) as outlined by the FDA and ICH regulations. It emphasizes the necessity for stability data from pilot and/or small scale batches, including 6 months of accelerated and long-term conditions, and details submission protocols and expectations for manufacturing and packaging processes. Additionally, it highlights that compliance with these guidelines is essential for the approval of drug substances and products under the FDA's regulatory framework.