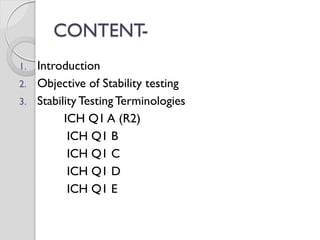
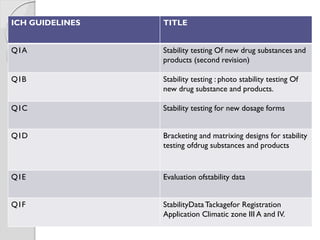
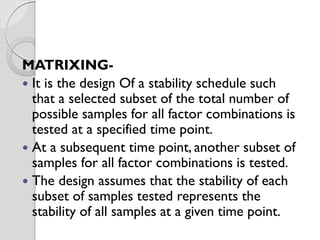
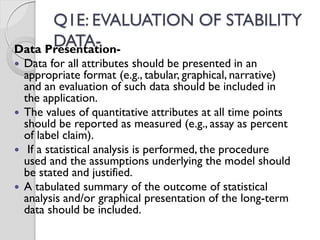
The document discusses ICH guidelines for stability testing of drug substances and products. The ICH guidelines provide recommendations for conducting stability testing to establish recommended storage conditions and shelf lives. Key ICH guidelines covered include Q1A on stability testing of new drug substances and products, Q1B on photostability testing, Q1C on stability testing for new dosage forms, and Q1E on evaluation of stability data. The guidelines address good stability practices such as stress testing, selection of batches, testing frequency, storage conditions and evaluation of results.