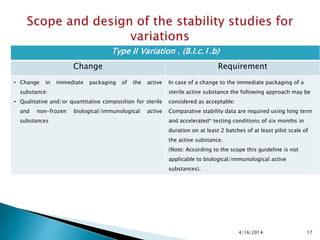
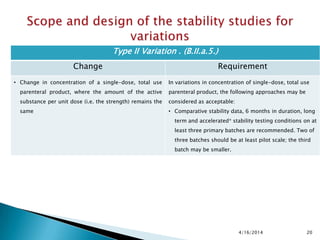
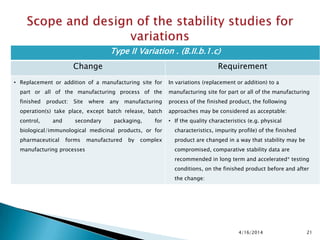
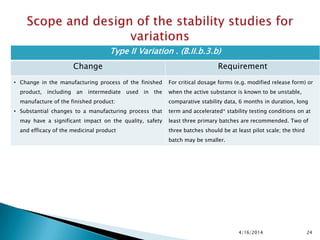
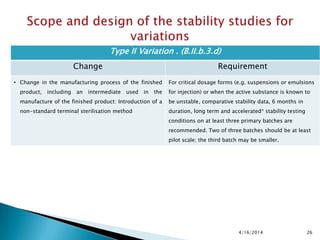
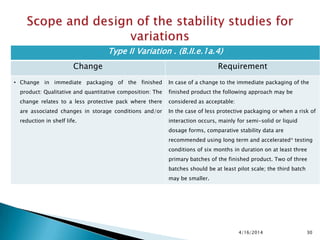
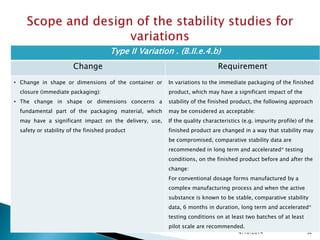
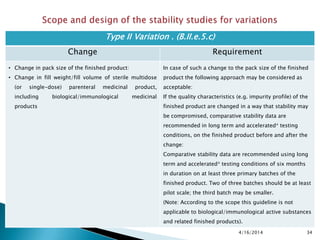
The presentation outlines stability testing requirements for variations to marketing authorizations in the pharmaceutical industry, provided by the non-profit organization Drug Regulations. It details different types of variations (Type I and Type II) and the associated stability data required for active substances and finished products, emphasizing the importance of demonstrating that changes do not impact product quality or safety. This resource serves as a guide for pharmaceutical professionals to ensure compliance with regulatory standards.