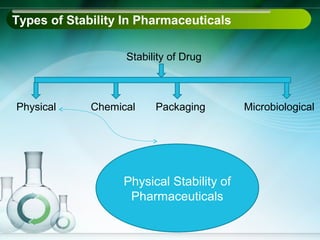
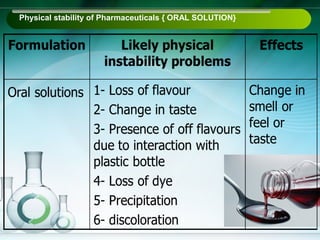
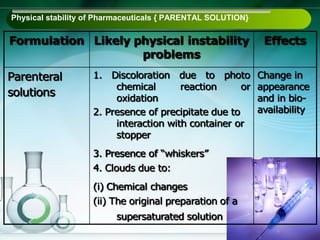
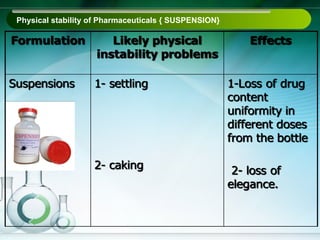
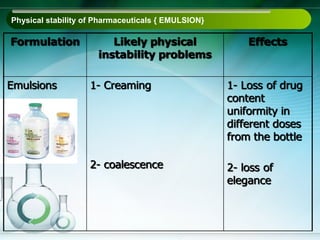
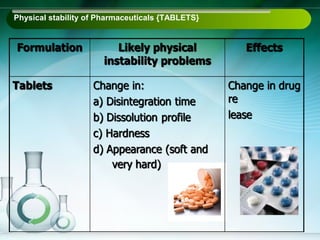
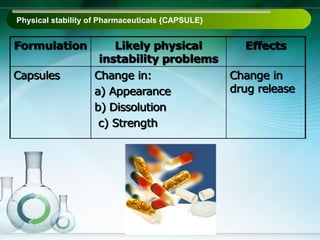
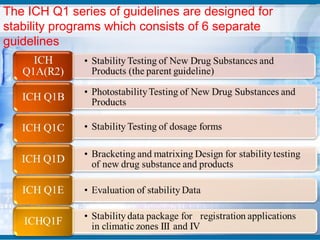
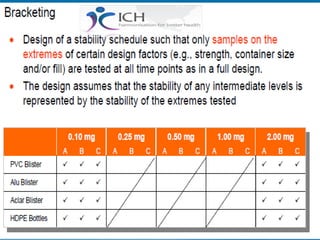
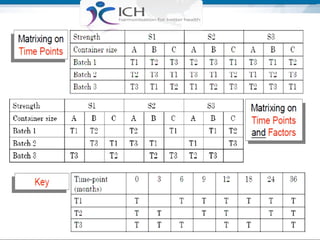
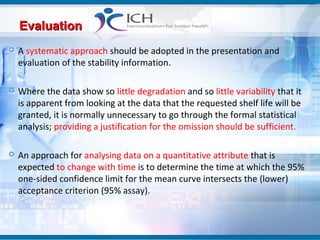
The document outlines the importance of stability studies in pharmaceuticals, summarizing definitions, requirements, and regulatory guidelines according to ICH standards. It discusses factors affecting drug stability, types of stability, and the need for comprehensive testing to ensure product quality and safety. Additionally, it emphasizes the structured approach to stability testing, including environmental impacts, formulation integrity, and the regulatory framework guiding these practices.