Embed presentation
Download as PDF, PPTX





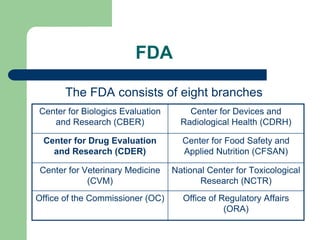




























The document outlines the key aspects of current good manufacturing practices (cGMPs) that pharmaceutical manufacturers must follow. cGMPs come from the Food, Drug and Cosmetic Act and are enforced by the FDA. They help ensure safety and quality by requiring strict control over facilities, equipment, components, packaging, labeling, and processes. Key parts of cGMP regulations address organization, buildings, equipment, materials control, production, packaging, holding, distribution, and records. Failure to comply can result in serious legal and business consequences like product recalls or plant shutdowns.

































