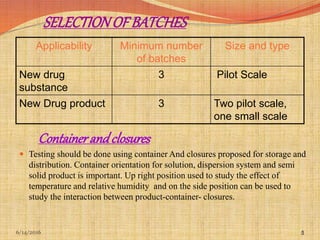
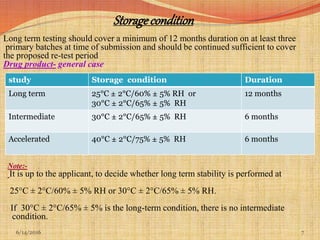
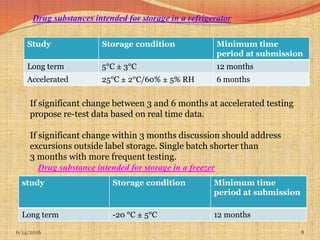
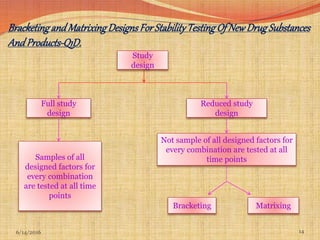
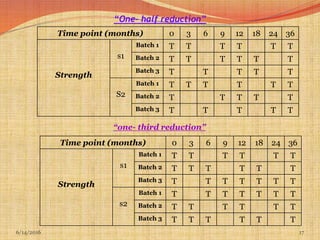
This document provides an overview of ICH guidelines for stability studies. It discusses guidelines Q1A-Q1F which provide recommendations for conducting stability testing of new drug substances and products. The guidelines address objectives, scope, general principles and recommendations for testing parameters, storage conditions, batch selection, specification setting and evaluation of stability data. Bracketing and matrixing designs are also covered as approaches to reduce the number of stability test samples required.