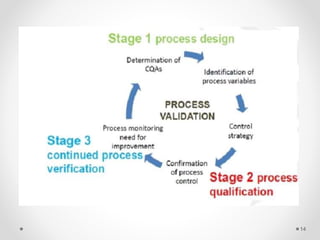
The document summarizes the US FDA's 2011 guidance on process validation, which outlines a lifecycle approach. It discusses the three stages of process validation according to the guidance: (1) Process Design which defines the commercial process based on development, (2) Process Qualification which evaluates the process's capability for commercial manufacturing, and (3) Continued Process Verification which gains ongoing assurance that the process remains in control during routine production. The lifecycle approach integrates validation strategies from previous guidelines and emphasizes continual process improvement, understanding sources of variation, and controlling variation to ensure consistent quality.