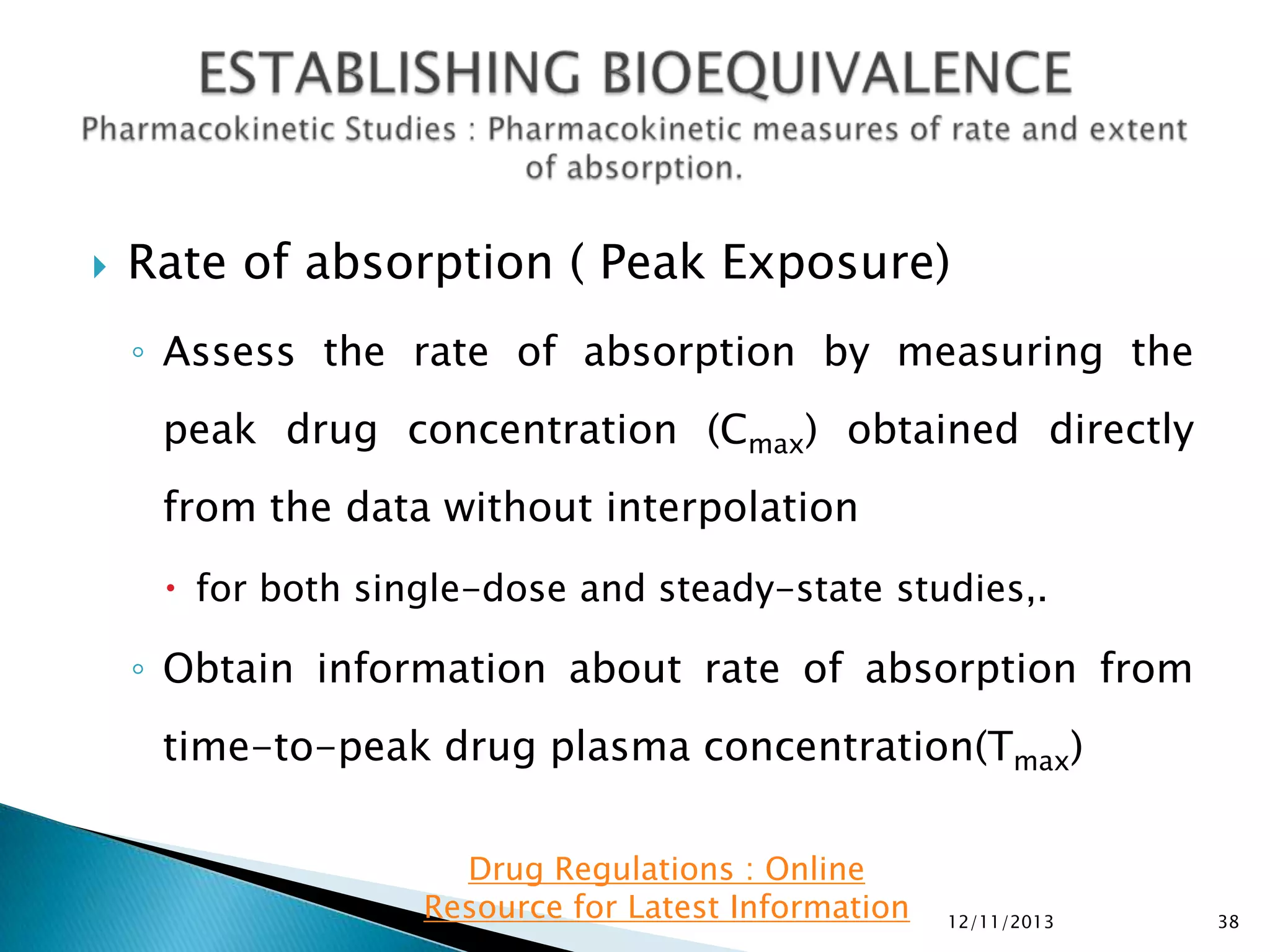
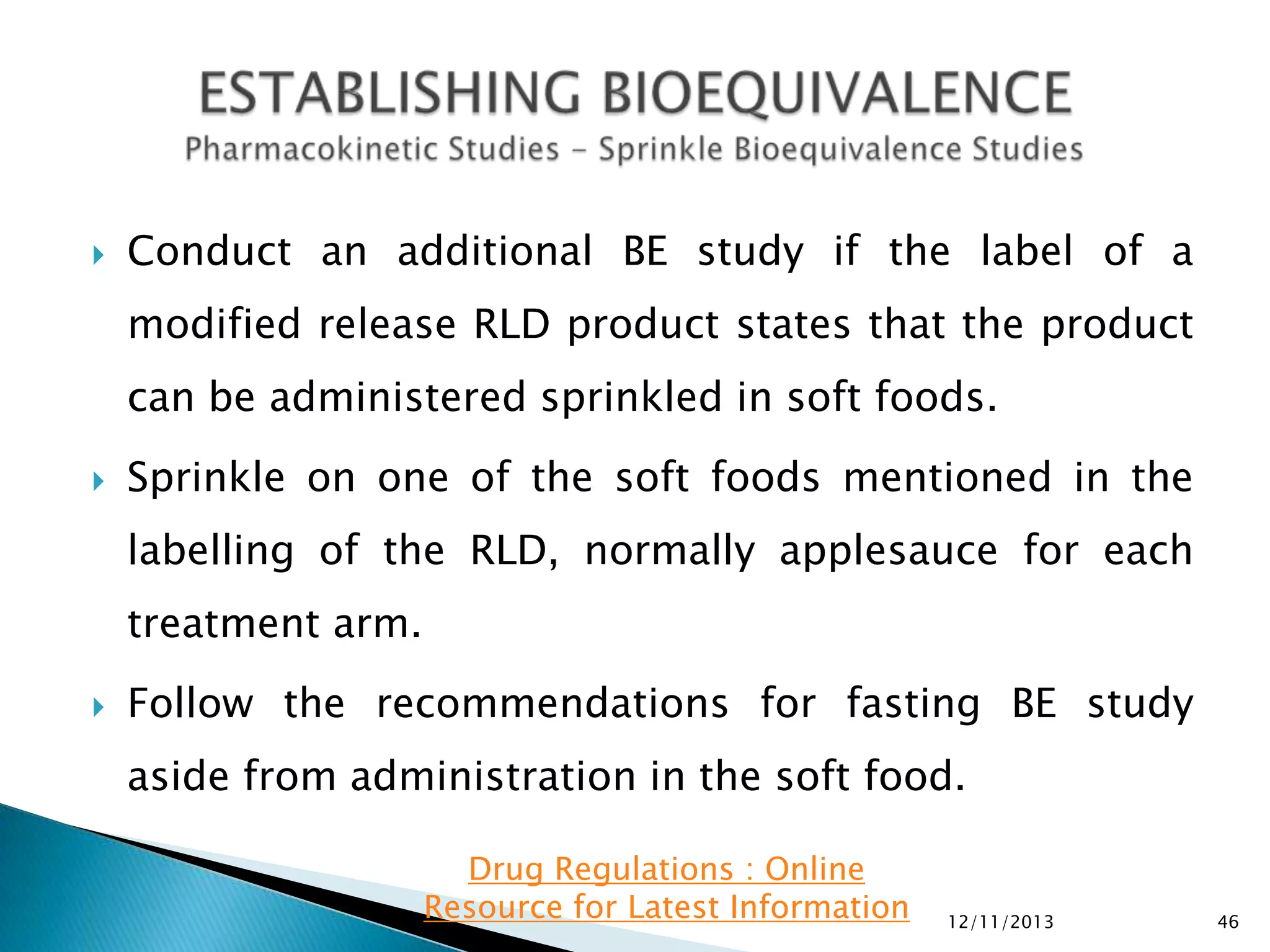
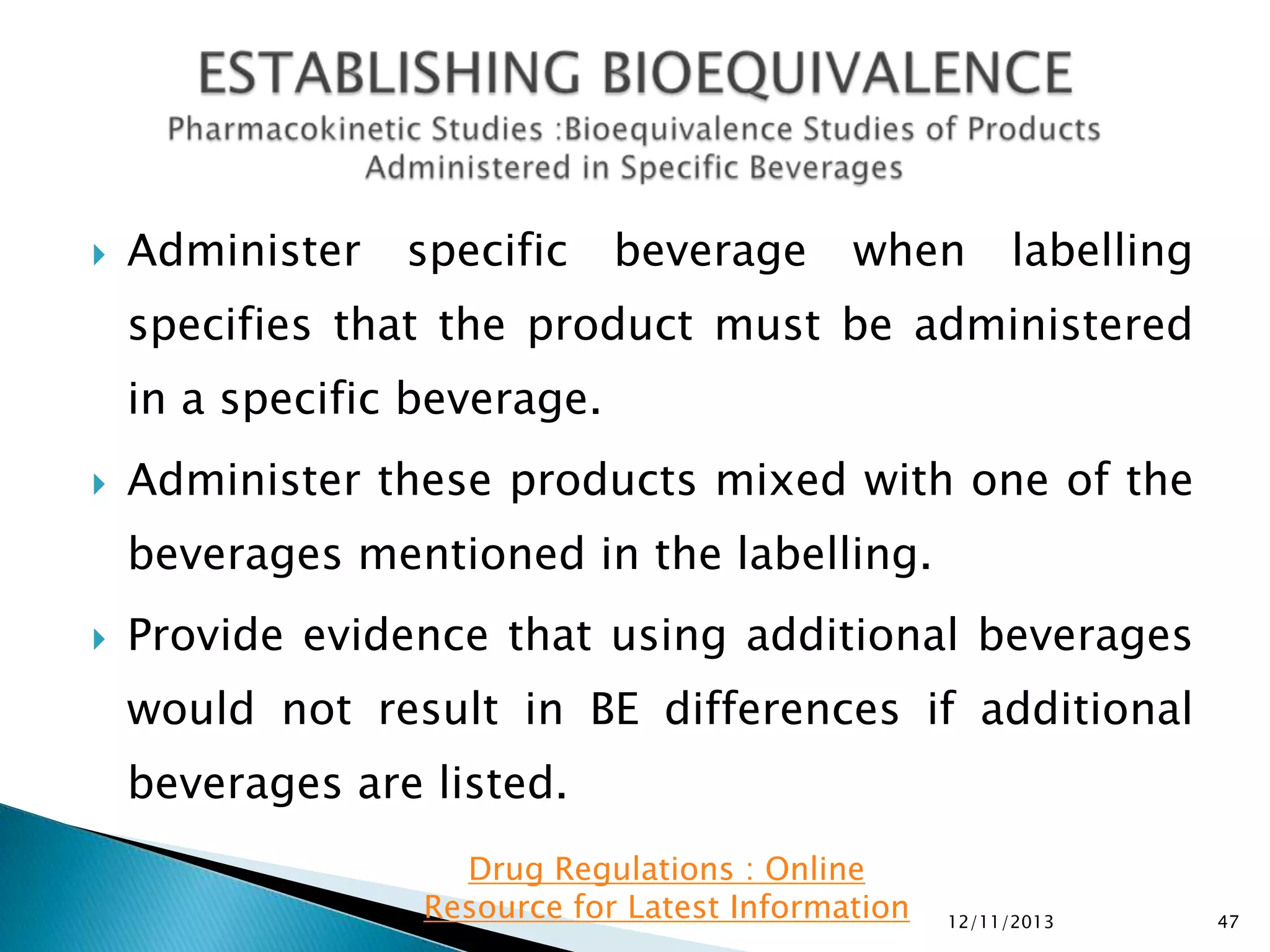
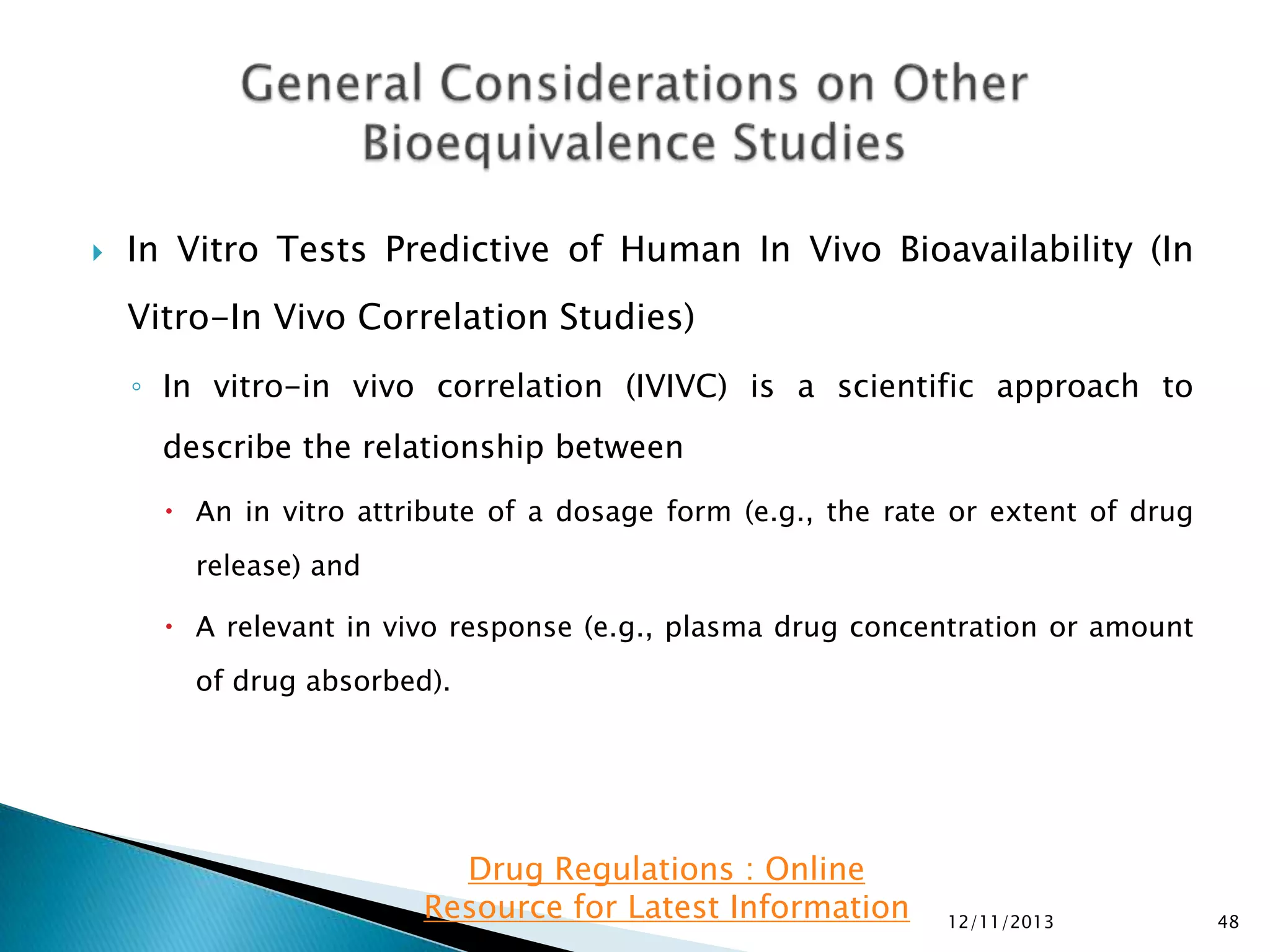
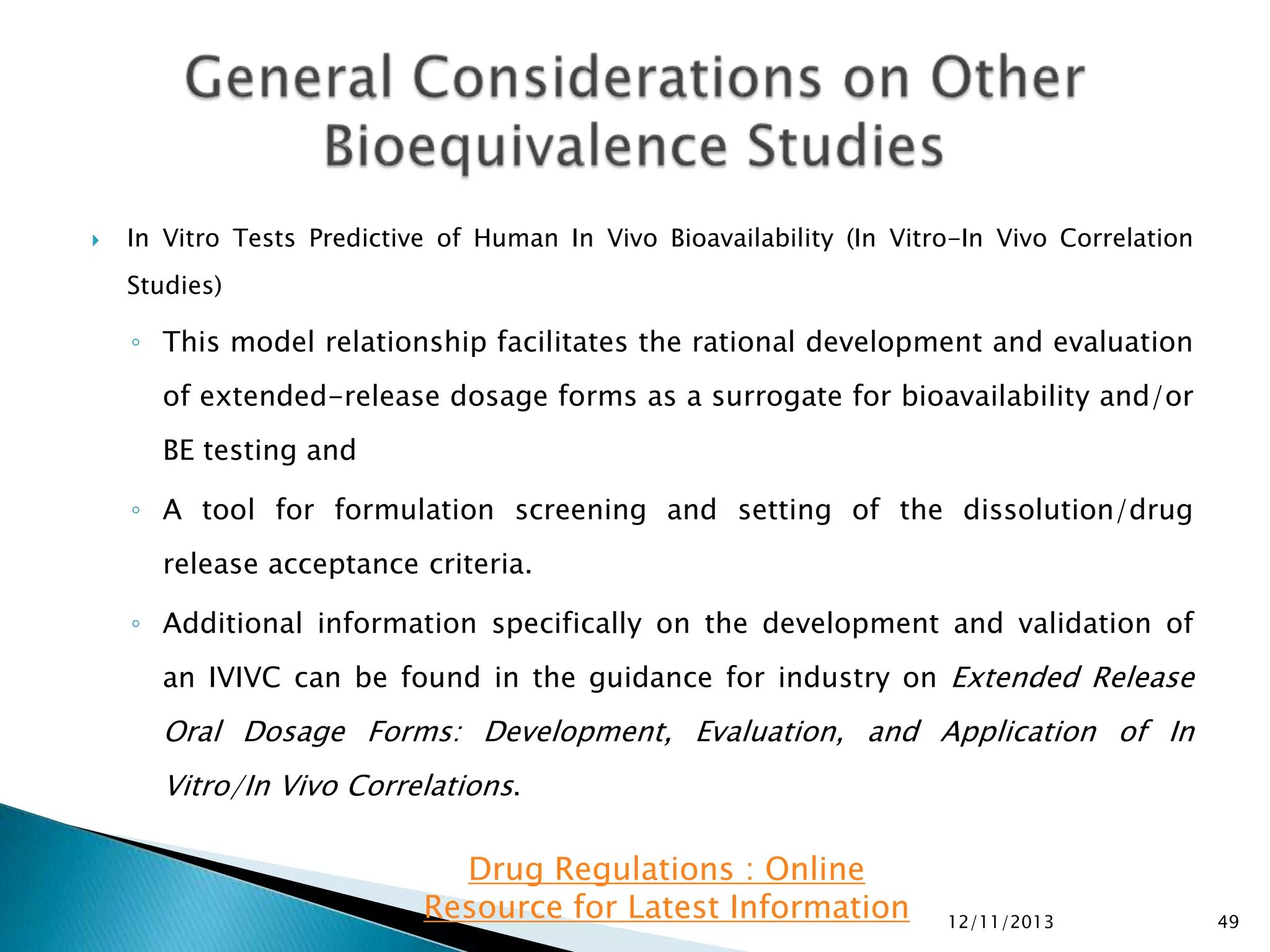
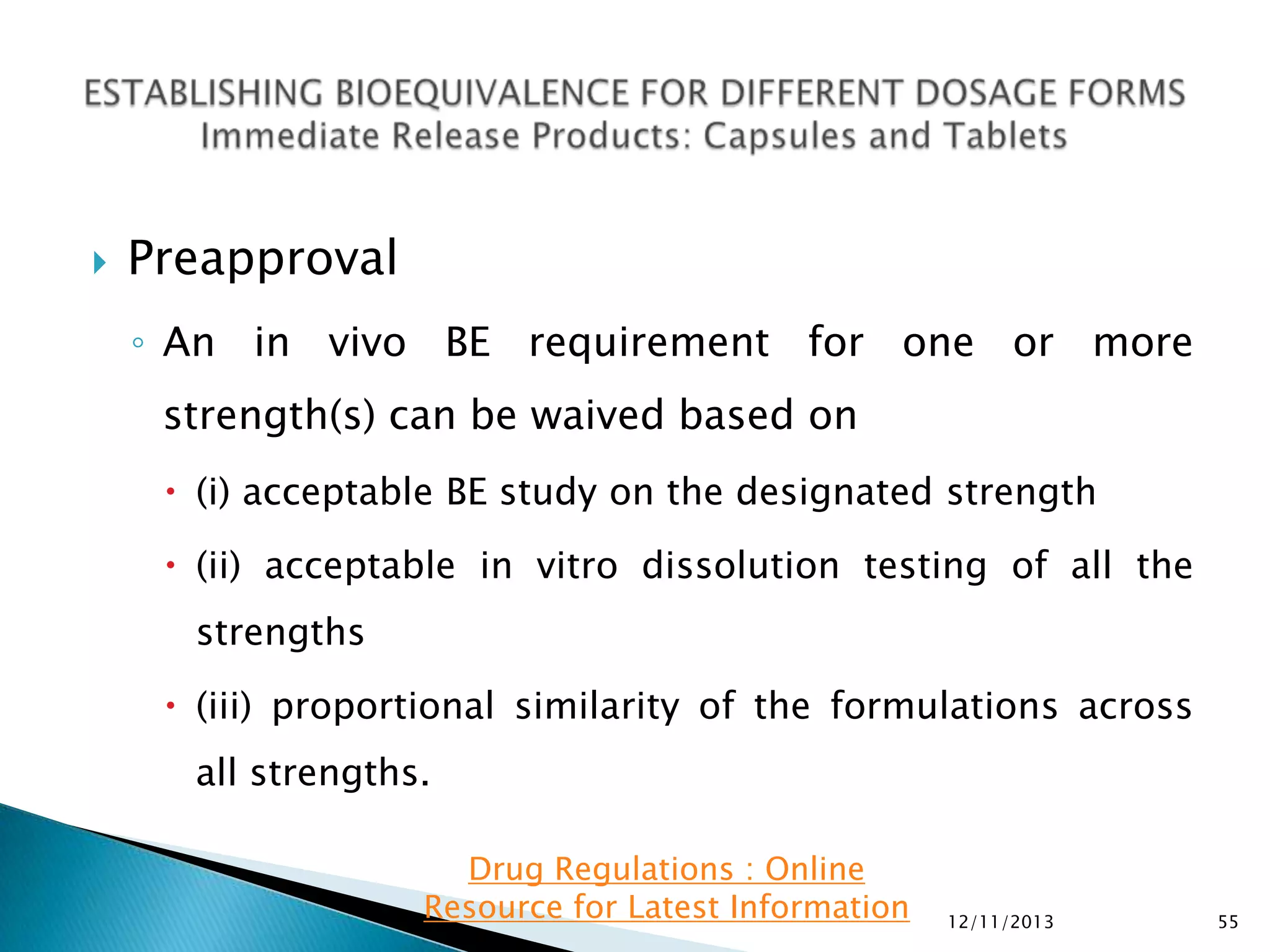
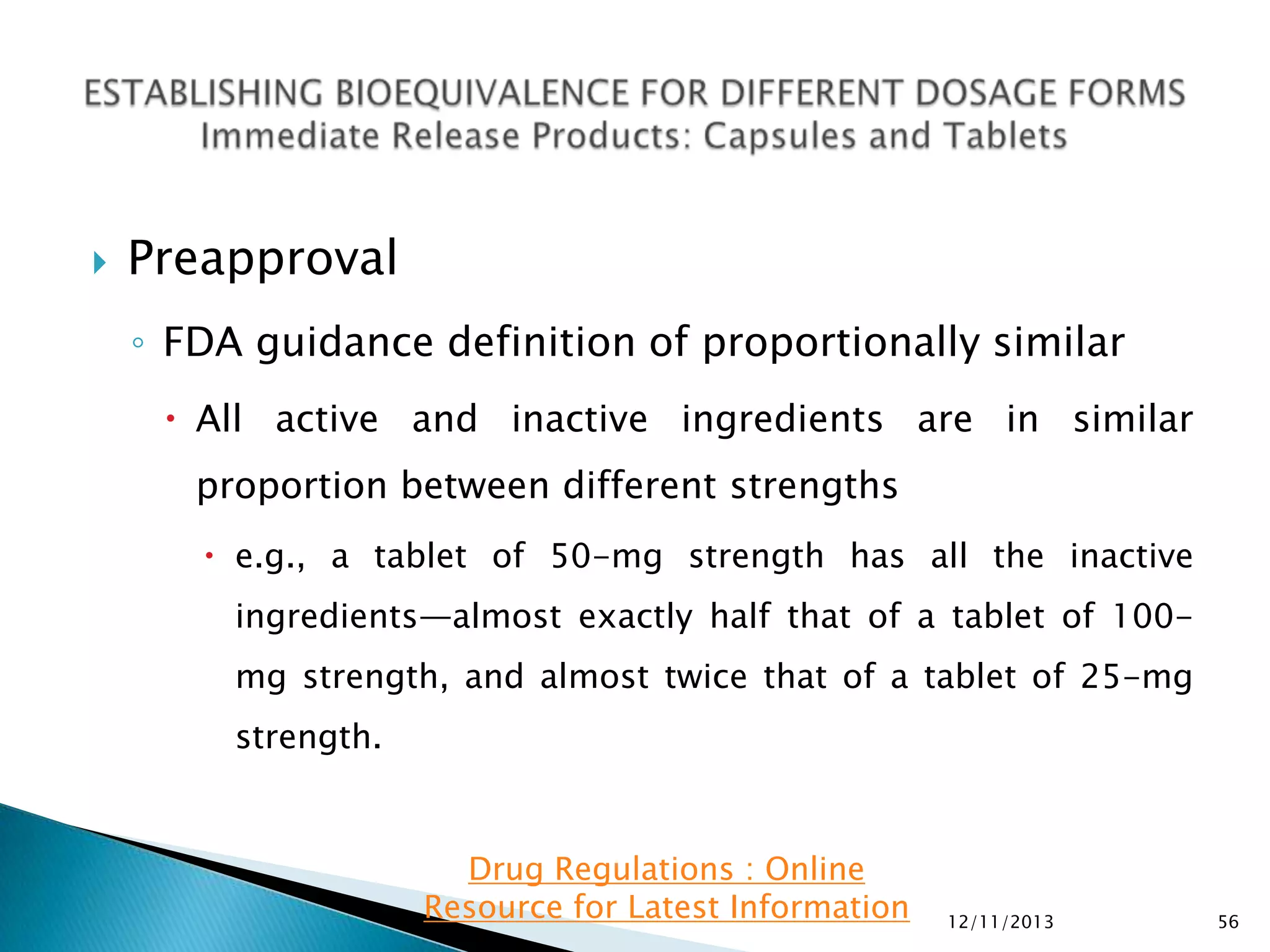
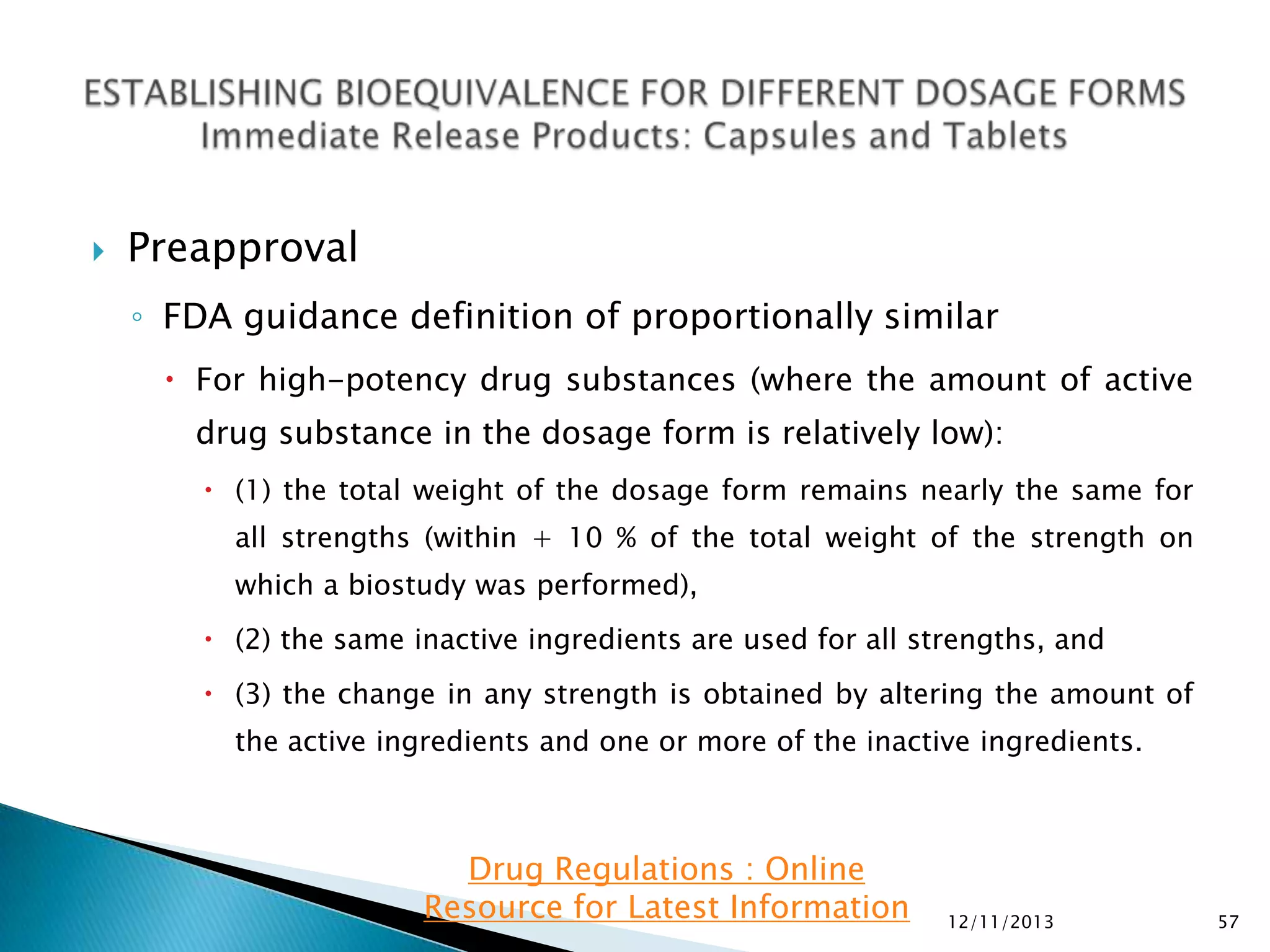
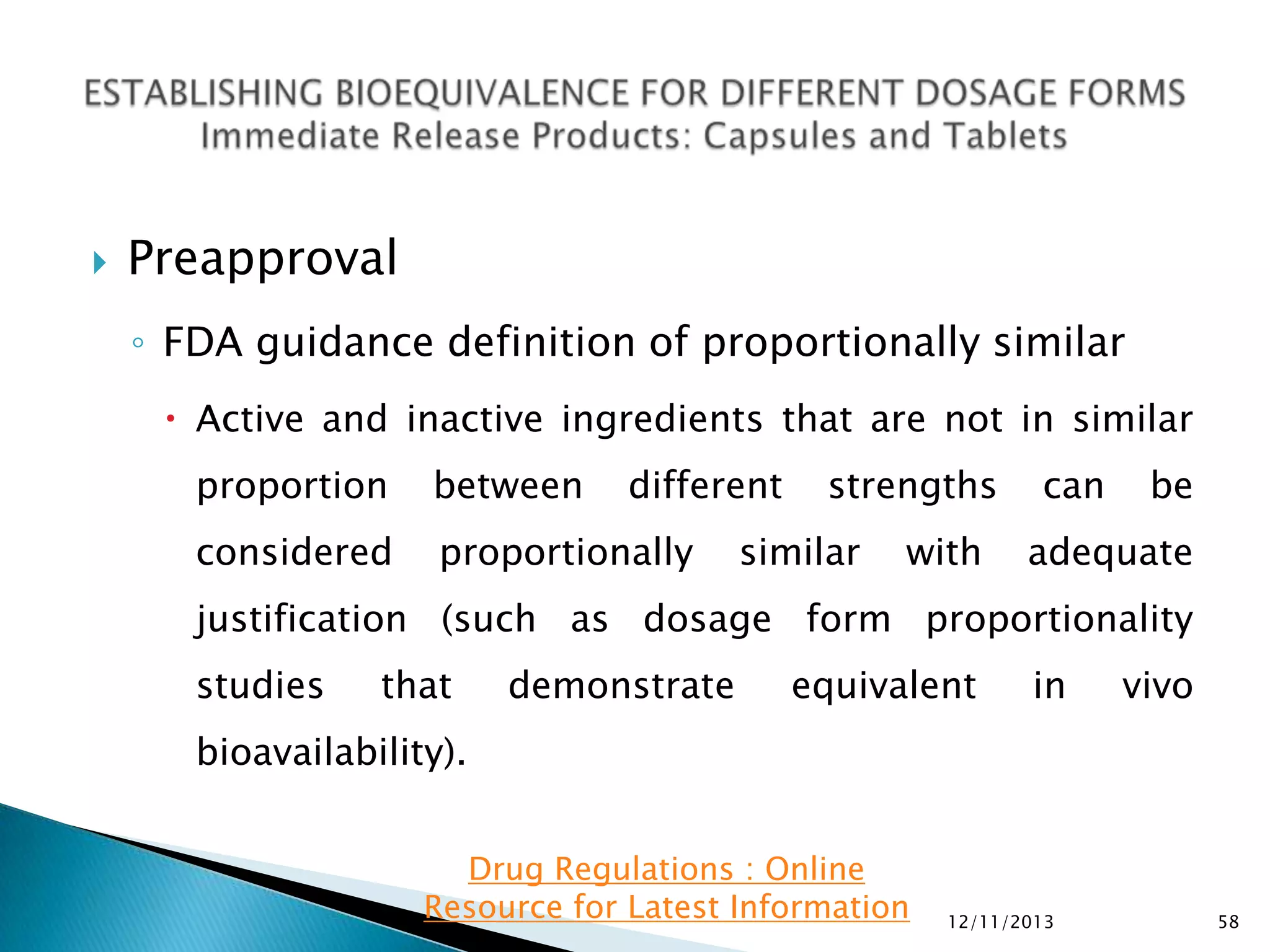
The document provides an overview of guidelines and recommendations by the non-profit organization 'Drug Regulations' for conducting bioequivalence (BE) studies in pharmaceutical research. It covers various aspects such as study design, sample collection, and statistical analysis to ensure the bioavailability of generic products compared to reference listed drugs. Detailed regulations and FDA references are included to assist professionals in the pharmaceutical field.


![
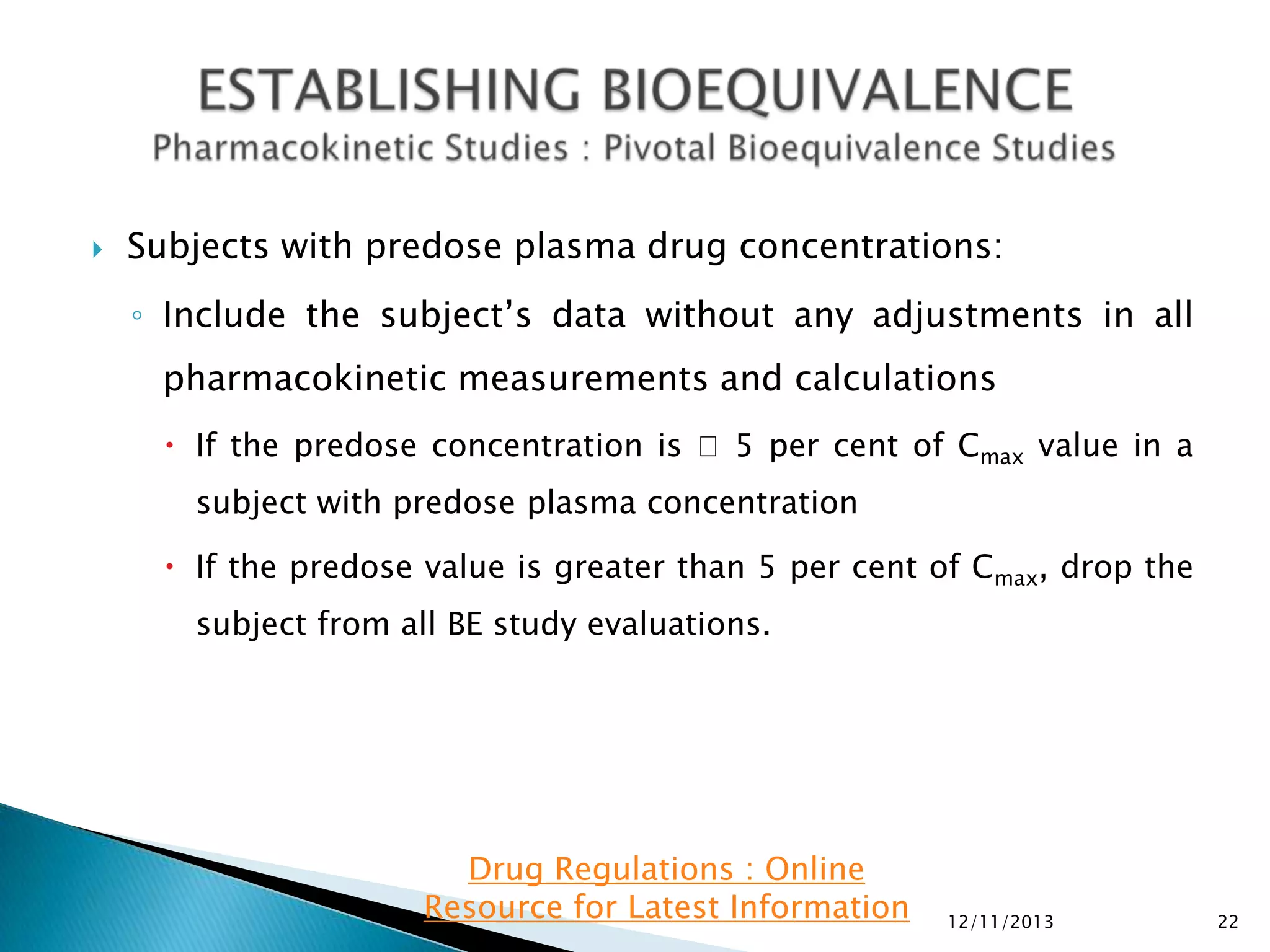
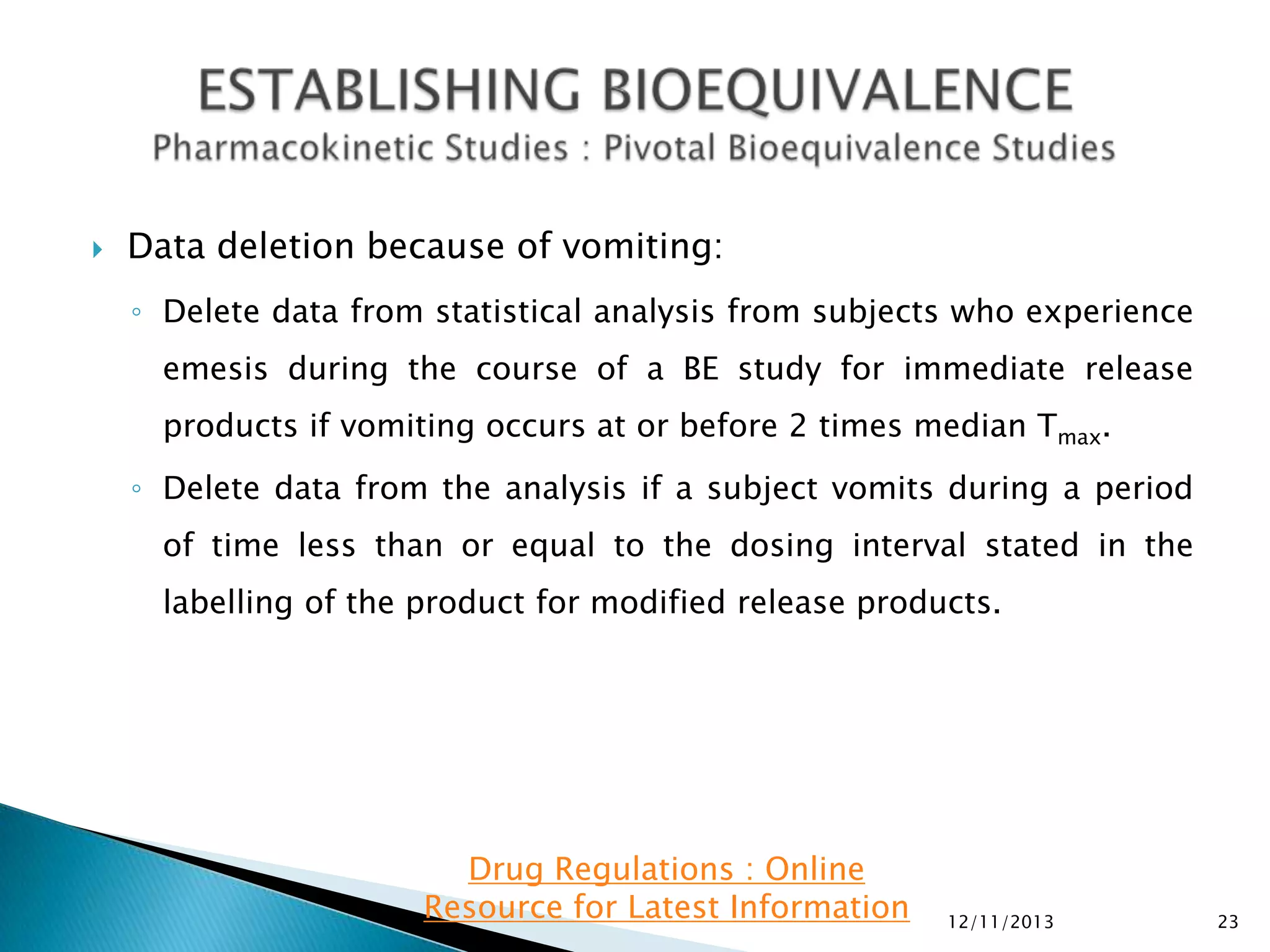
Pharmacokinetic information in submissions:
◦ Plasma concentrations and time points
◦ Subject, period, sequence, treatment
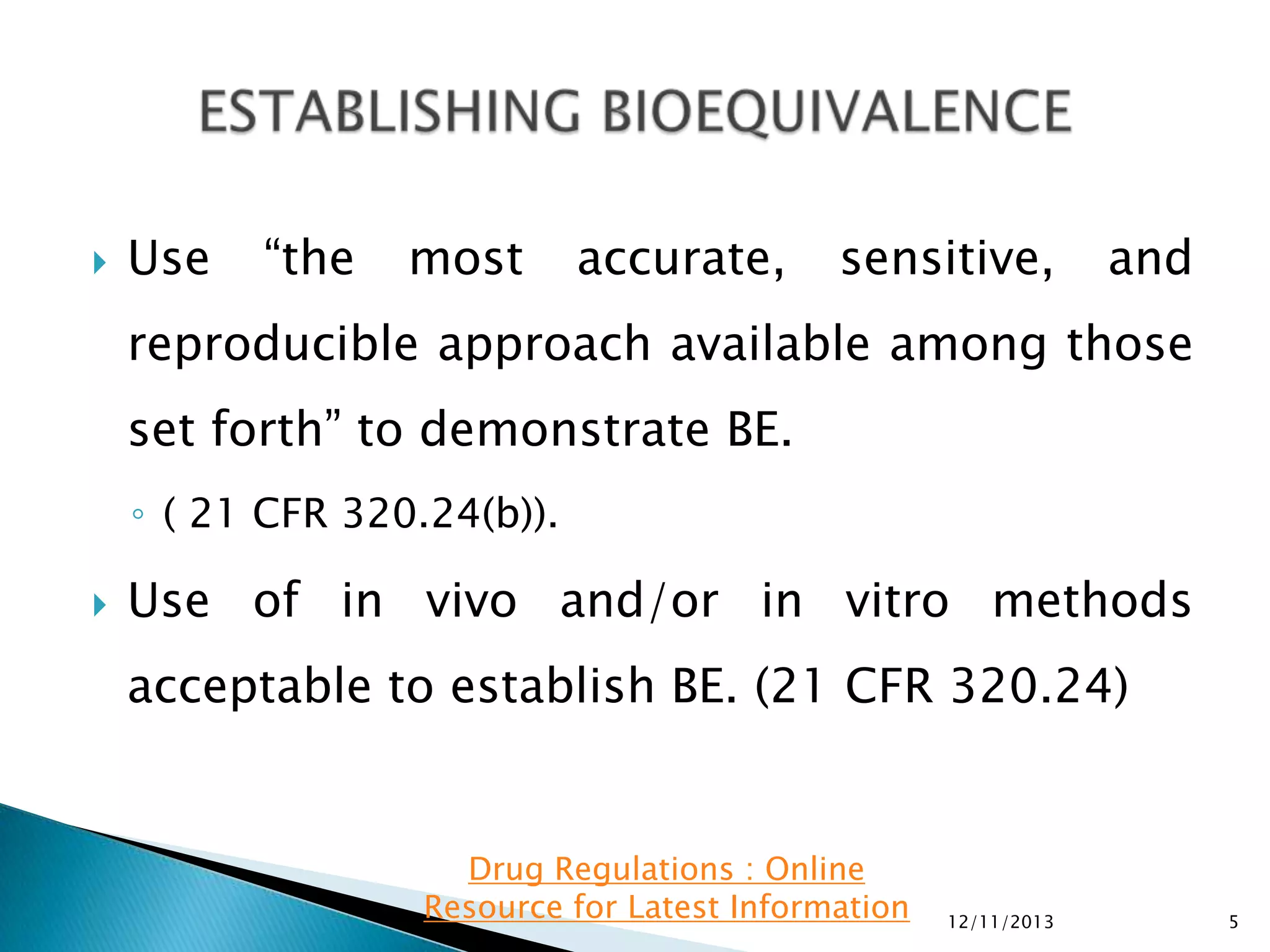
◦ Intersubject, intrasubject, and/or total variability, if available
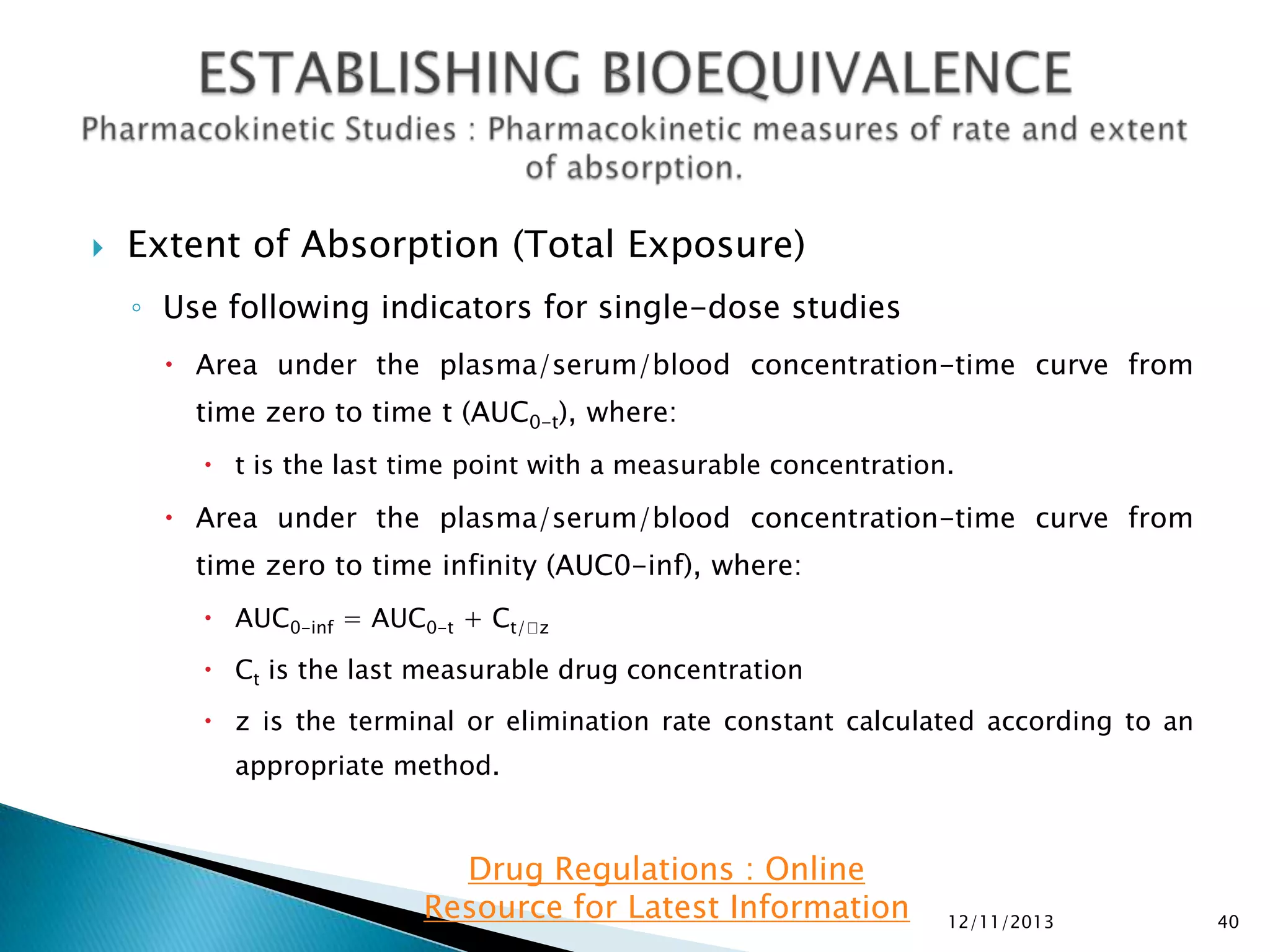
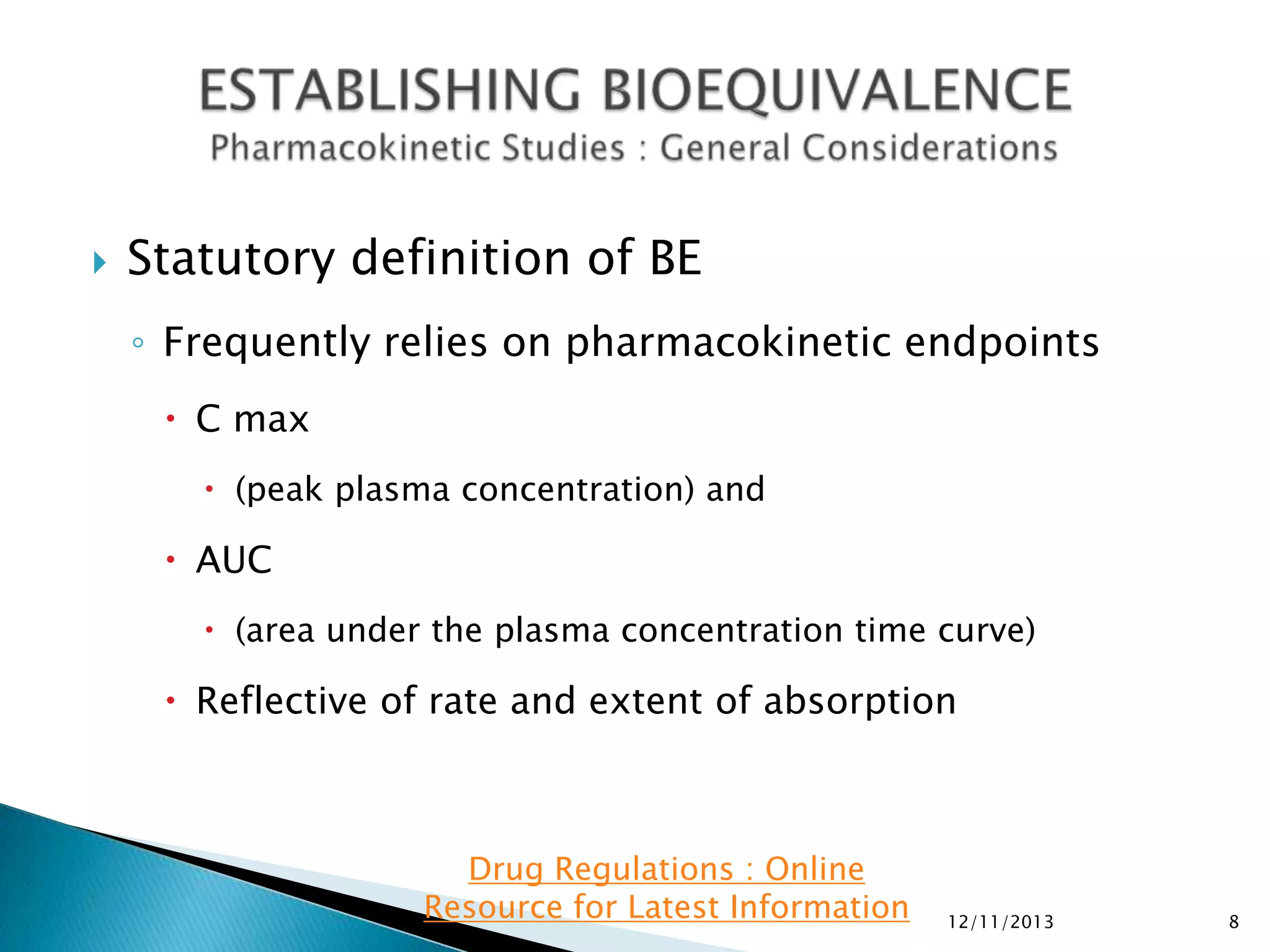
◦ For single-dose BE studies:
AUC0-t, AUC0-inf, and C
max.
In addition, report the following supportive information: Tmax, Kel and t1/2.
◦ For steady-state BE studies: AUC0-tau and CmaxSS. In addition, please report
CminSS (concentration at the end of a dosing interval), CavSS (average
concentration during a dosing interval), degree of fluctuation [(CmaxCmin)/CavSS], swing [(CmaxSS-CminSS)/CminSS], and Tmax.
Drug Regulations : Online
Resource for Latest Information
12/11/2013
24](https://image.slidesharecdn.com/newguidanceonbiorquivalence-131211224504-phpapp02/75/New-guidance-on-Bioequivalence-24-2048.jpg)