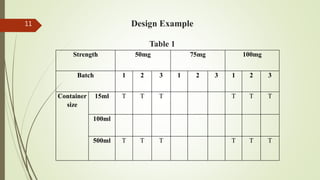
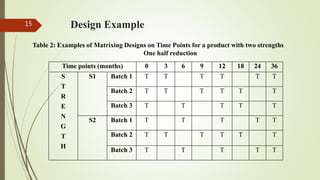
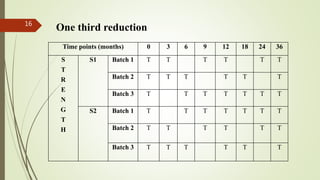
This document discusses bracketing and matrixing designs for stability testing of new drug substances and products according to ICH Q1D guidelines. Bracketing design involves testing only the extremes of design factors like strength or container size, assuming stability of intermediates is represented by extremes. Matrixing design involves testing selected combinations of factors at each time point rather than all combinations at all time points. Both designs provide reduced testing compared to full design testing all samples at all time points, but require justification and carry potential risks of underestimating shelf life if variability is high.