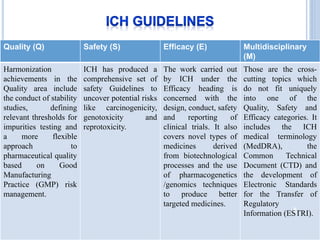
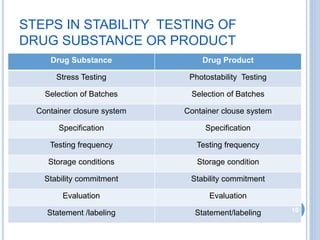
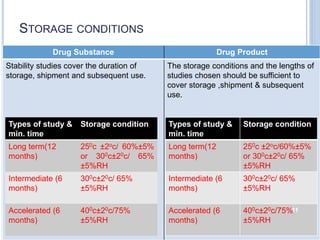
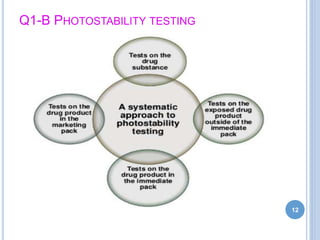
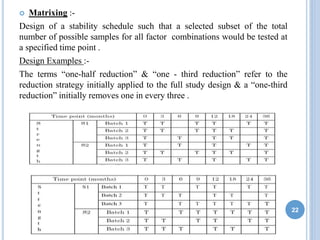
The document discusses International Council for Harmonisation (ICH) guidelines related to stability testing of drug substances and products. It provides an overview of the historical background and development of ICH. It summarizes several ICH guidelines including Q1A on stability testing, Q1B on photostability testing, Q1C on stability testing for new dosage forms, and Q1D on bracketing and matrixing designs for stability testing. It also discusses stability storage conditions, principles of ICH guidelines for stability testing, and the objectives of guidelines like Q1E on evaluation of stability data.
















![REFERENCES
[1]Om M. Bagade, Textbook of pharmaceutical QUALITY
ASSURANCE First Edition, Career Publication. page No.220-256
[2] https://www.ich.org/
[3] C.V. S. Subrahmanyam, Pharmaceutical Regulatory Affairs ,Vallabh
Prakashan .page no. 182-199
[4]https://www.researchgate.net/publication/306312875_Adoptio
n_and_Reasons_for_Withdrawal_of_ICH_Q1F_Guidelines
[5]Dr. Ruchi Tiwari,Dr.Gaurav Tiwari,Intellactual Property Rights &
Drug Regulatory Affairs,Nirali Prakashan,Page No.23.1-23.12
[6]Vandana patravale.,John Disouza.,Maharukh
Rustomji,Pharmaceutical product Development CRC press Group
of taylor and francis. Page no.207-213.
26](https://image.slidesharecdn.com/drugstabilitycorrectedcopy-200126073245/85/Drug-stability-26-320.jpg)
