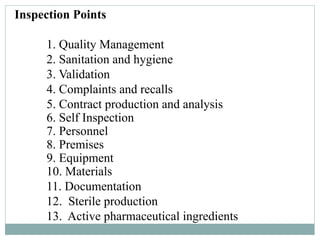
The document presents an overview of Good Manufacturing Practices (GMP), detailing its principles, certification process, and requirements by the WHO for pharmaceutical production. It emphasizes the importance of compliance and procedural documentation to ensure product quality and safety, particularly for international trade. Furthermore, it discusses inspection protocols, and the increasing demand for GMP certification among Indian pharmaceutical manufacturers due to rising exports.