Embed presentation
Downloaded 23 times










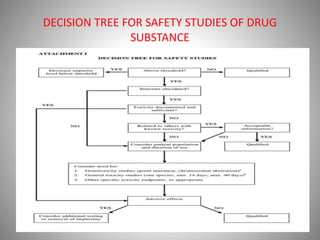


This document summarizes a seminar presentation on impurities and stability studies. It defines impurities as any component of a drug substance that is not the defined chemical entity. Impurities are classified as organic, inorganic, or residual solvents. Organic impurities can arise from manufacturing processes or storage and include starting materials, byproducts, and degradation products. Inorganic impurities result from manufacturing and include reagents, metals, and salts. Residual solvents are volatile organic chemicals used in drug substance synthesis. The document also discusses ICH guidelines for qualifying impurities based on safety testing and provides a decision tree for conducting safety studies of drug substances.












