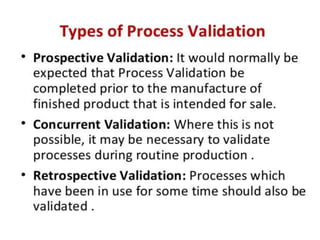
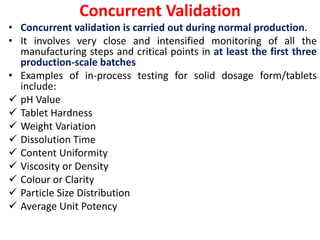
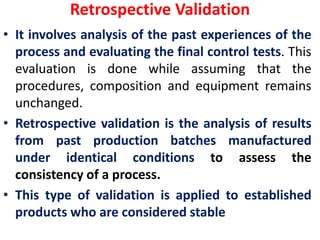
Validation ensures that processes and methods consistently produce results within specifications. Qualification proves equipment works correctly and leads to expected results through testing. Calibration compares instrument measurements to standards to ensure accuracy. A Validation Master Plan summarizes an organization's validation strategy and ensures resources are available for validation projects. It discusses validation activities across the organization.