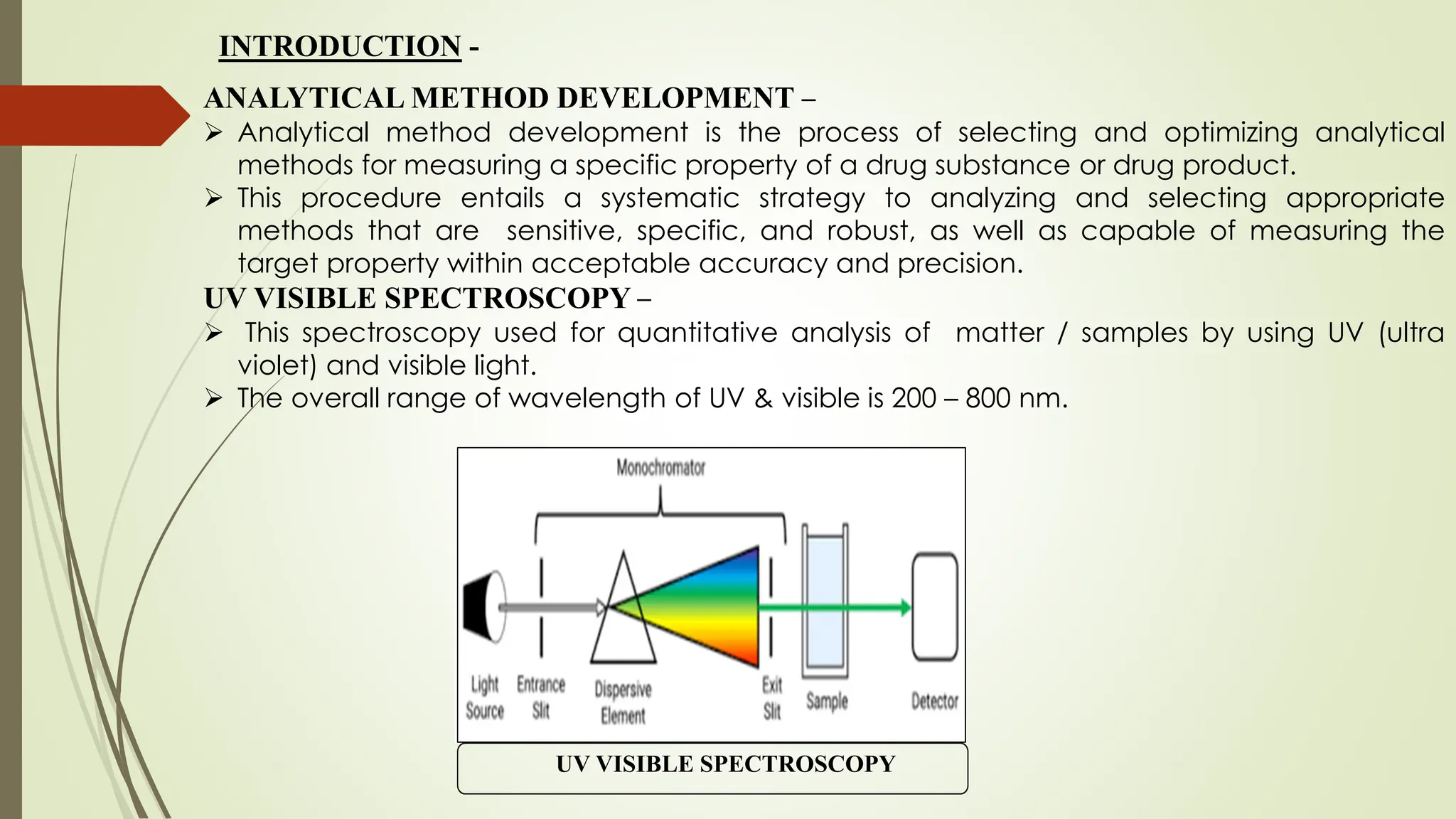
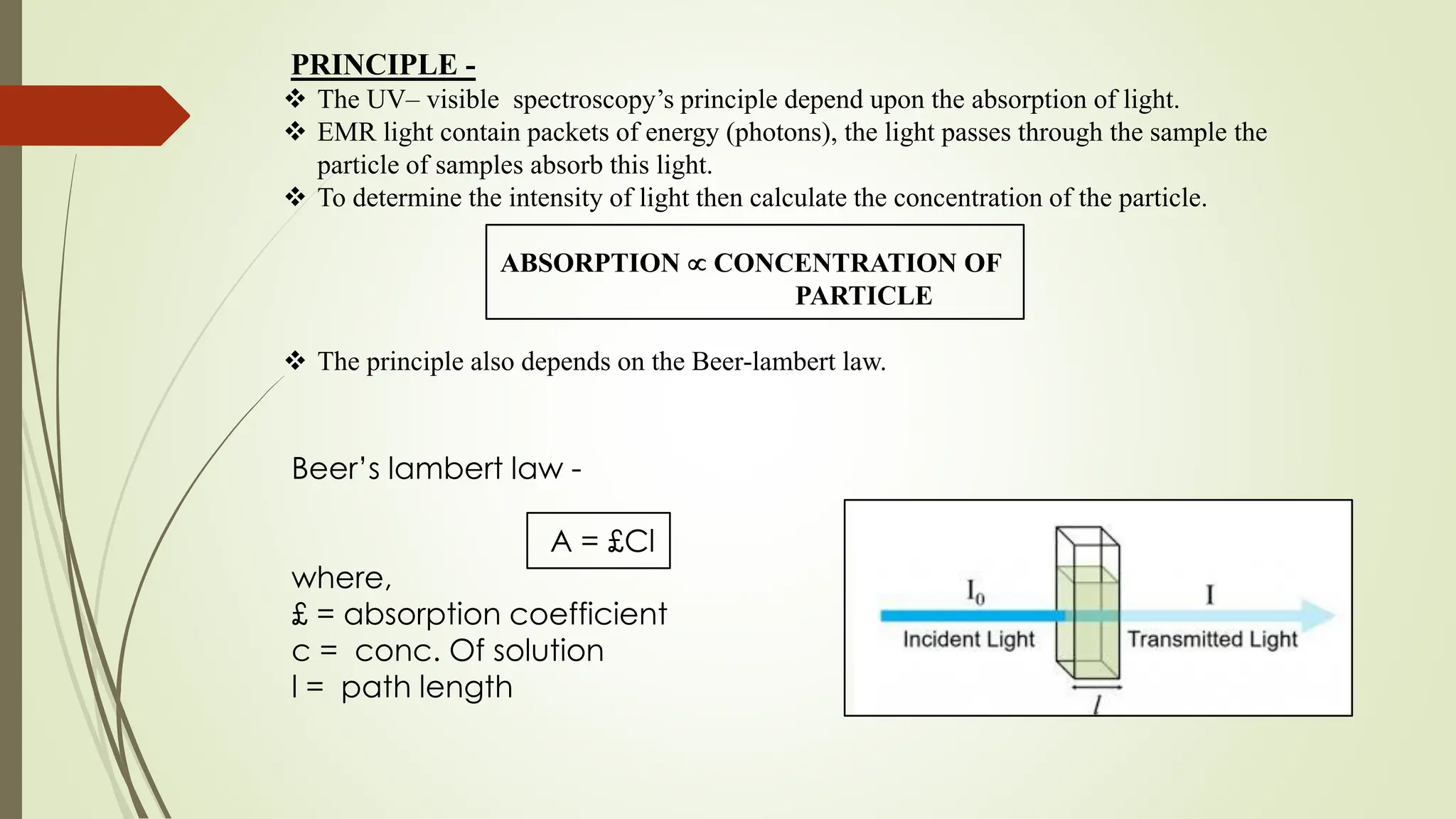
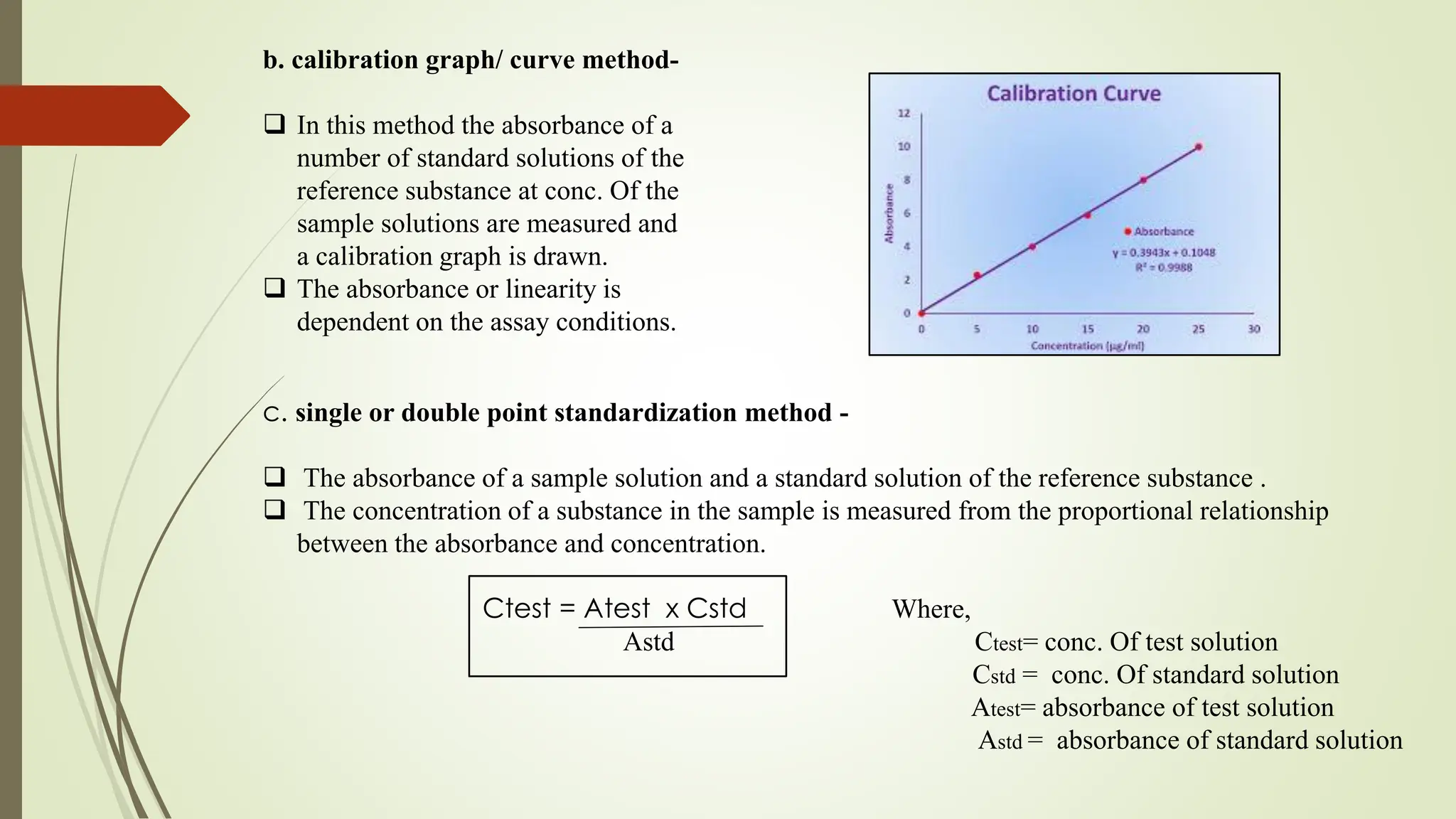
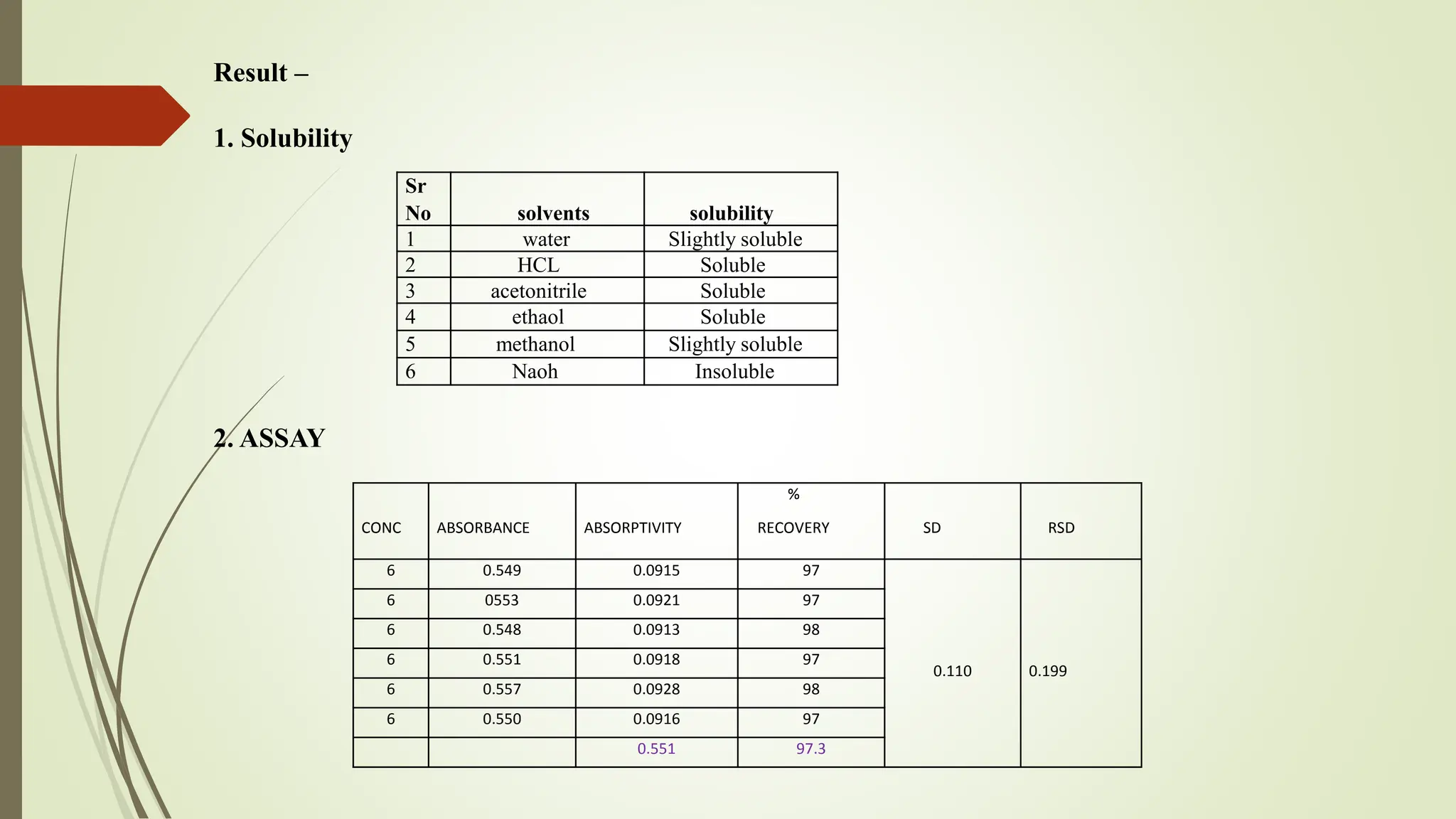
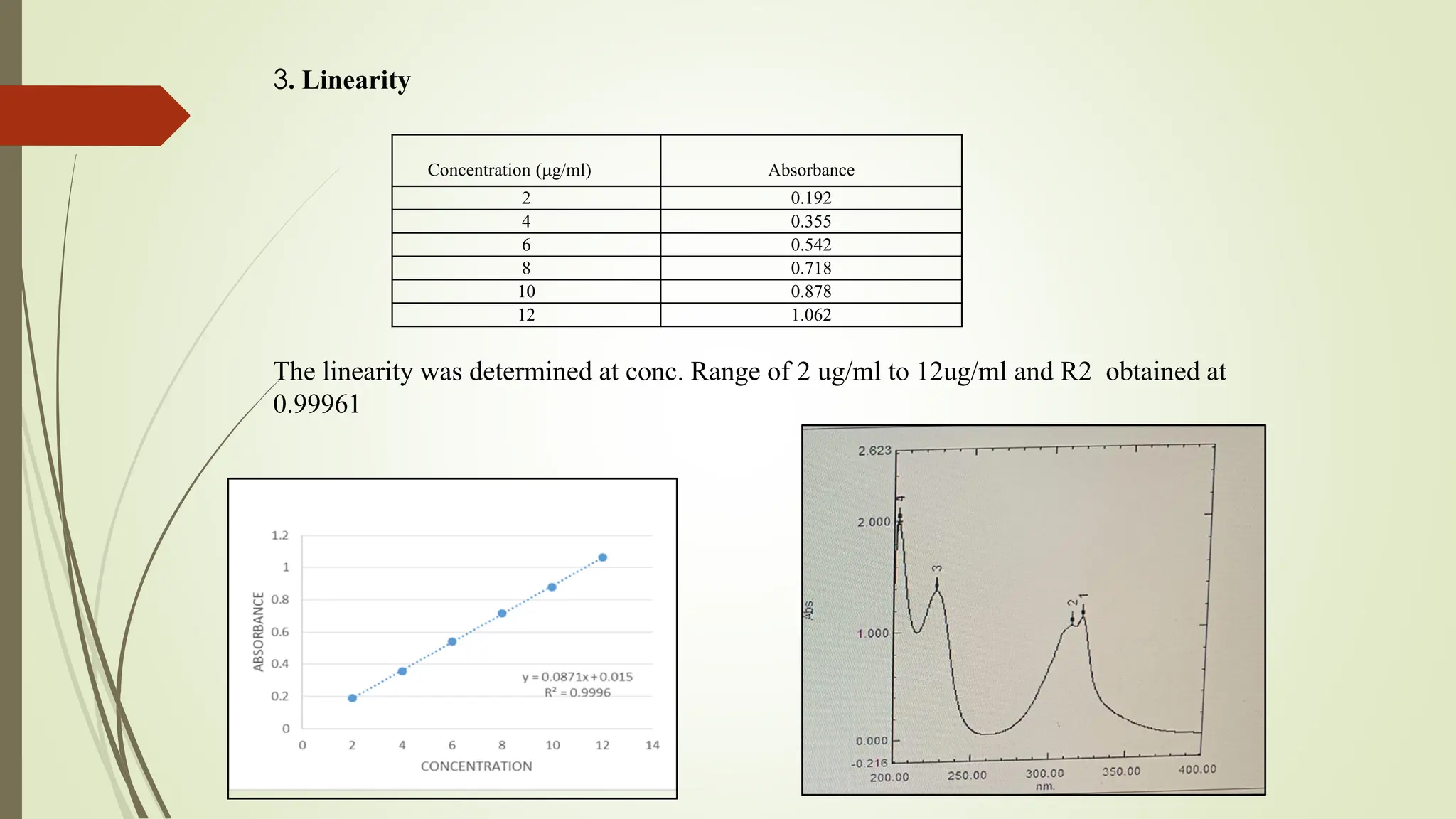
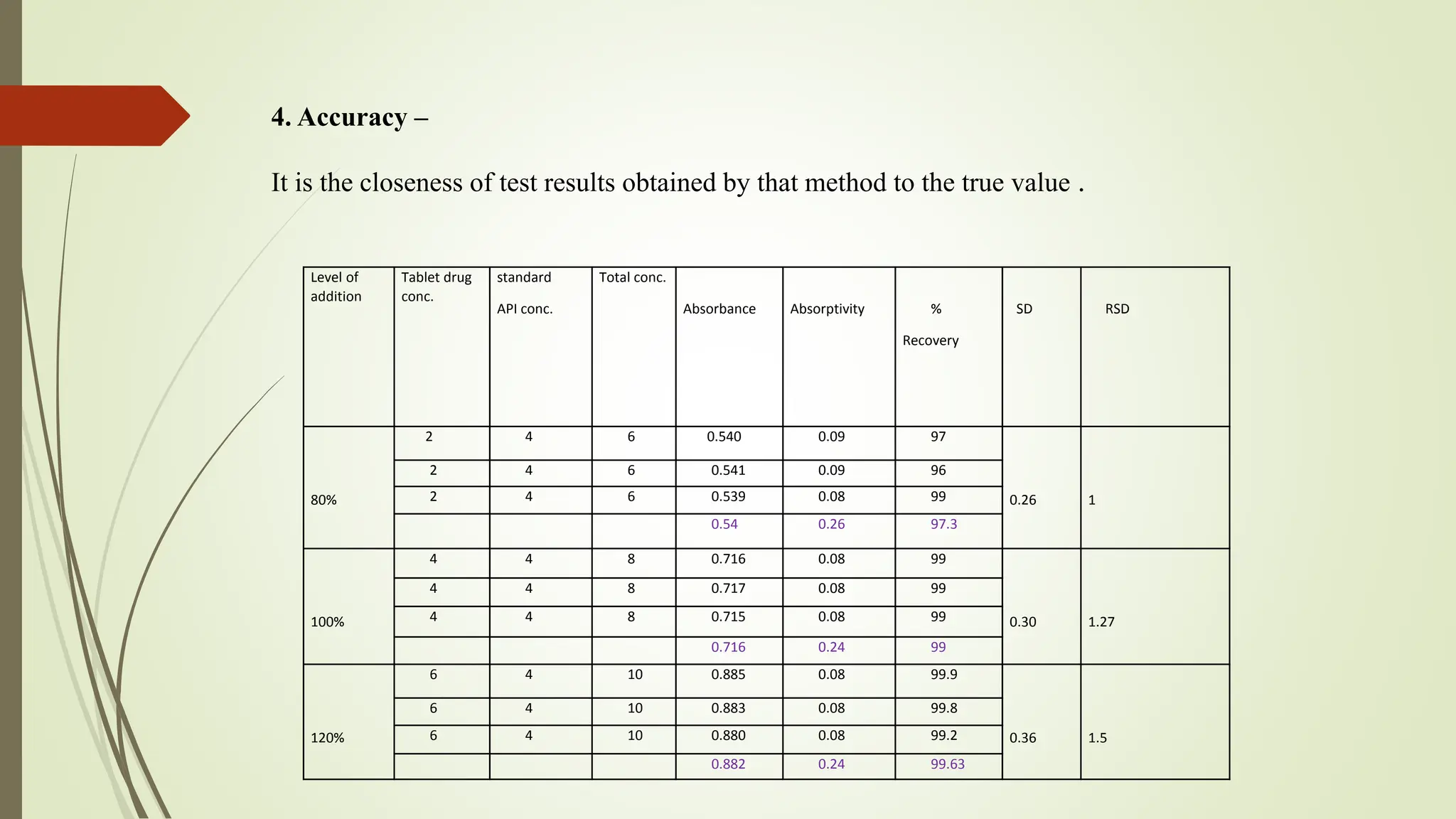
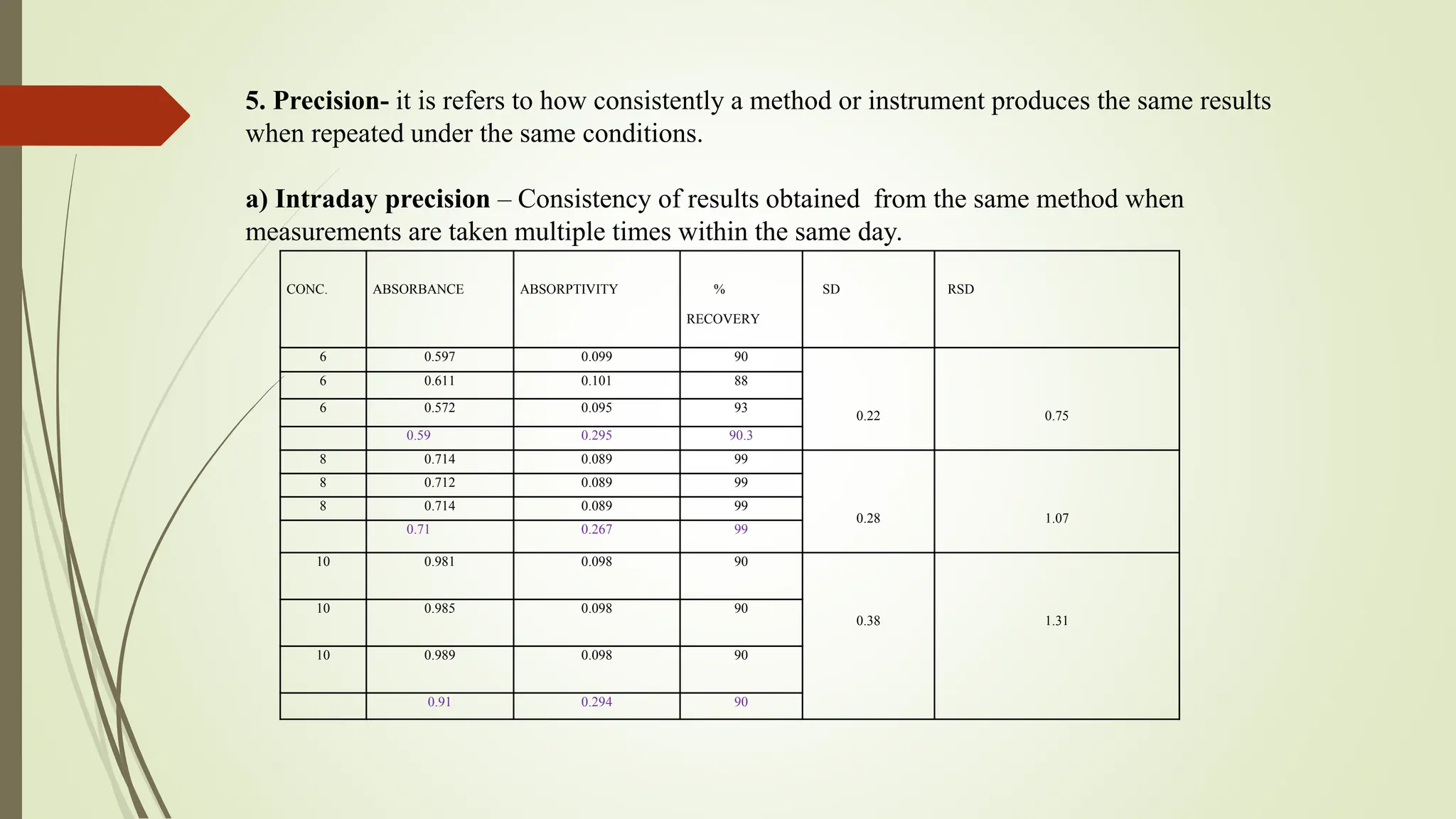
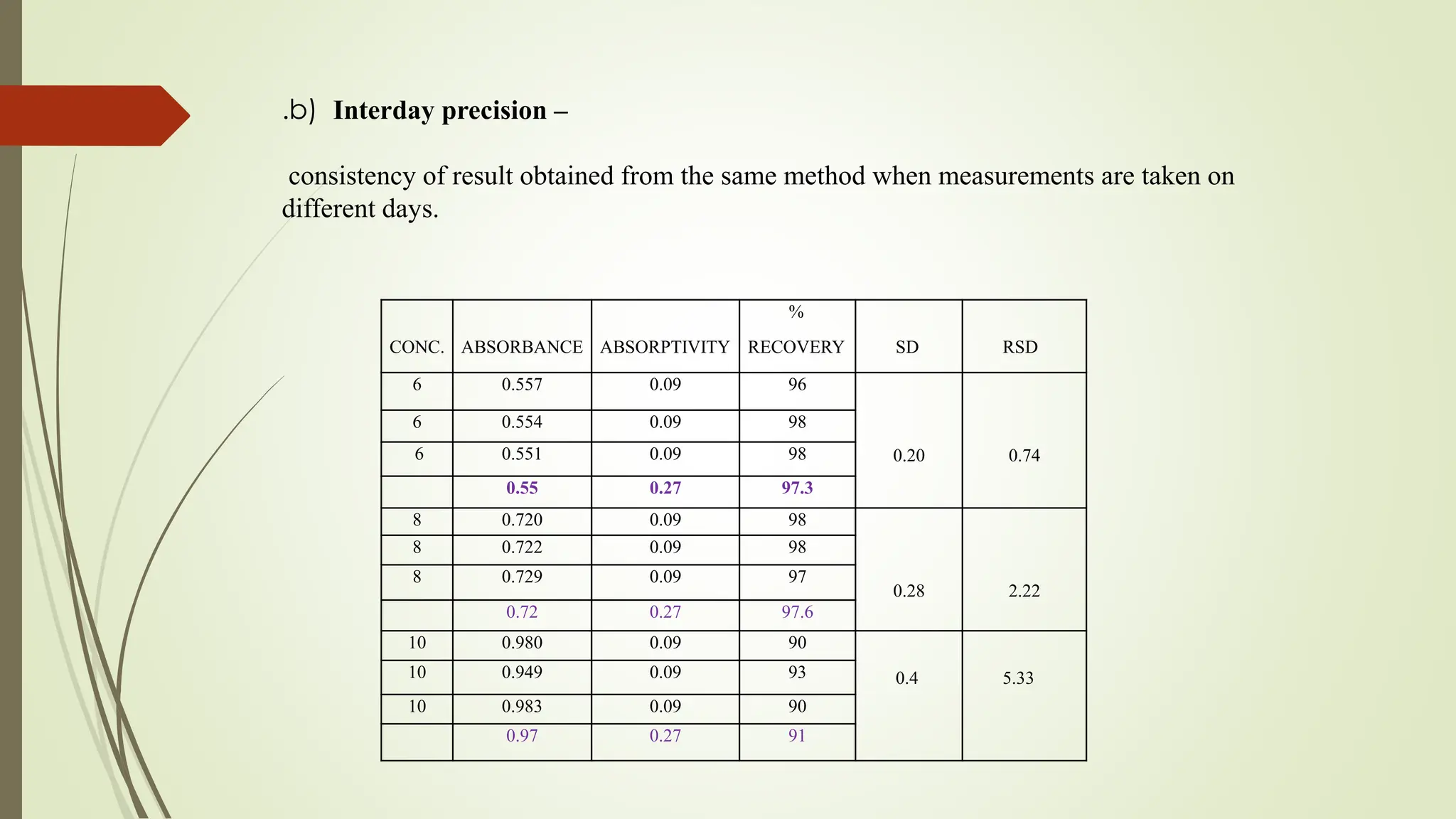
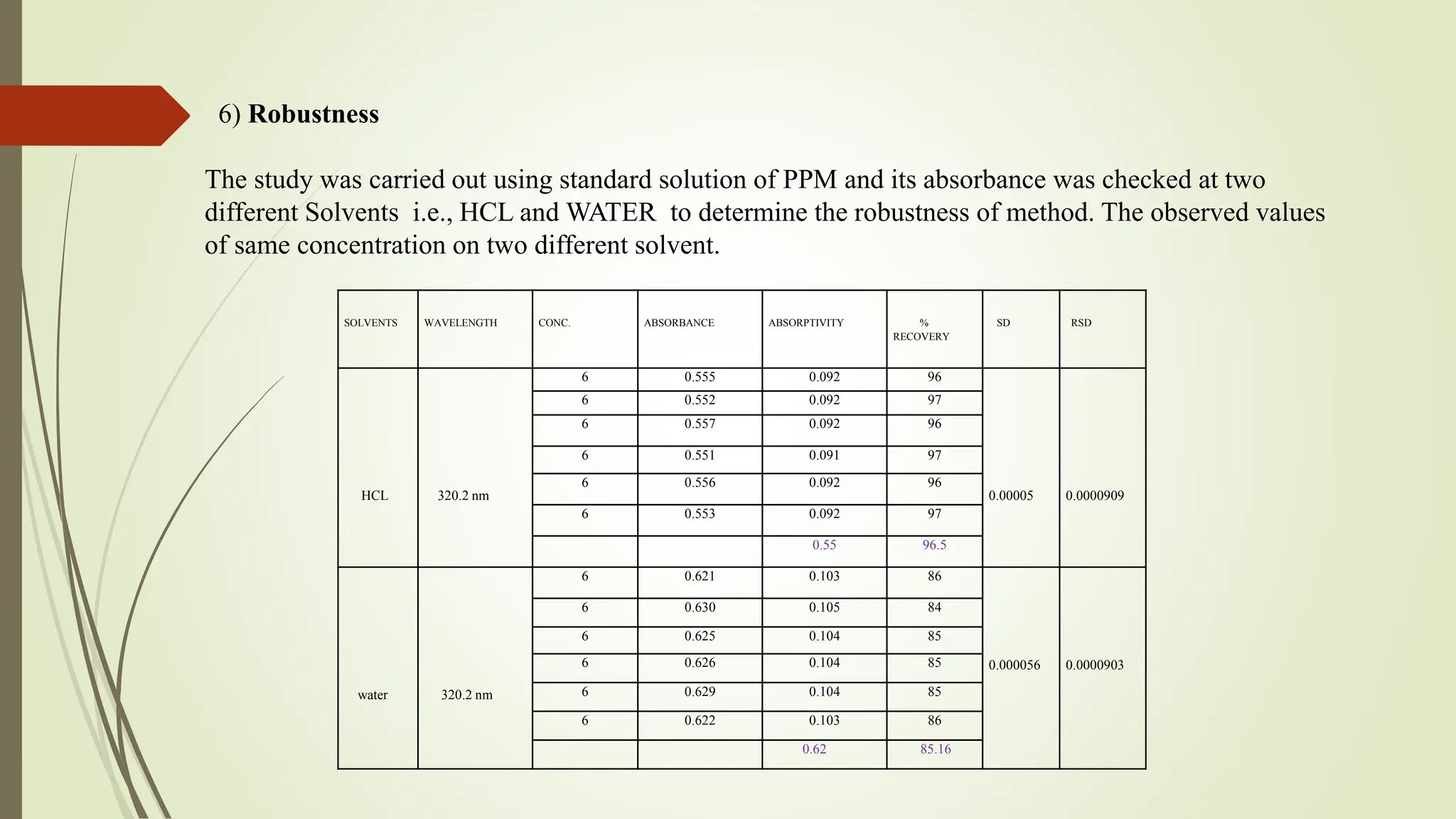
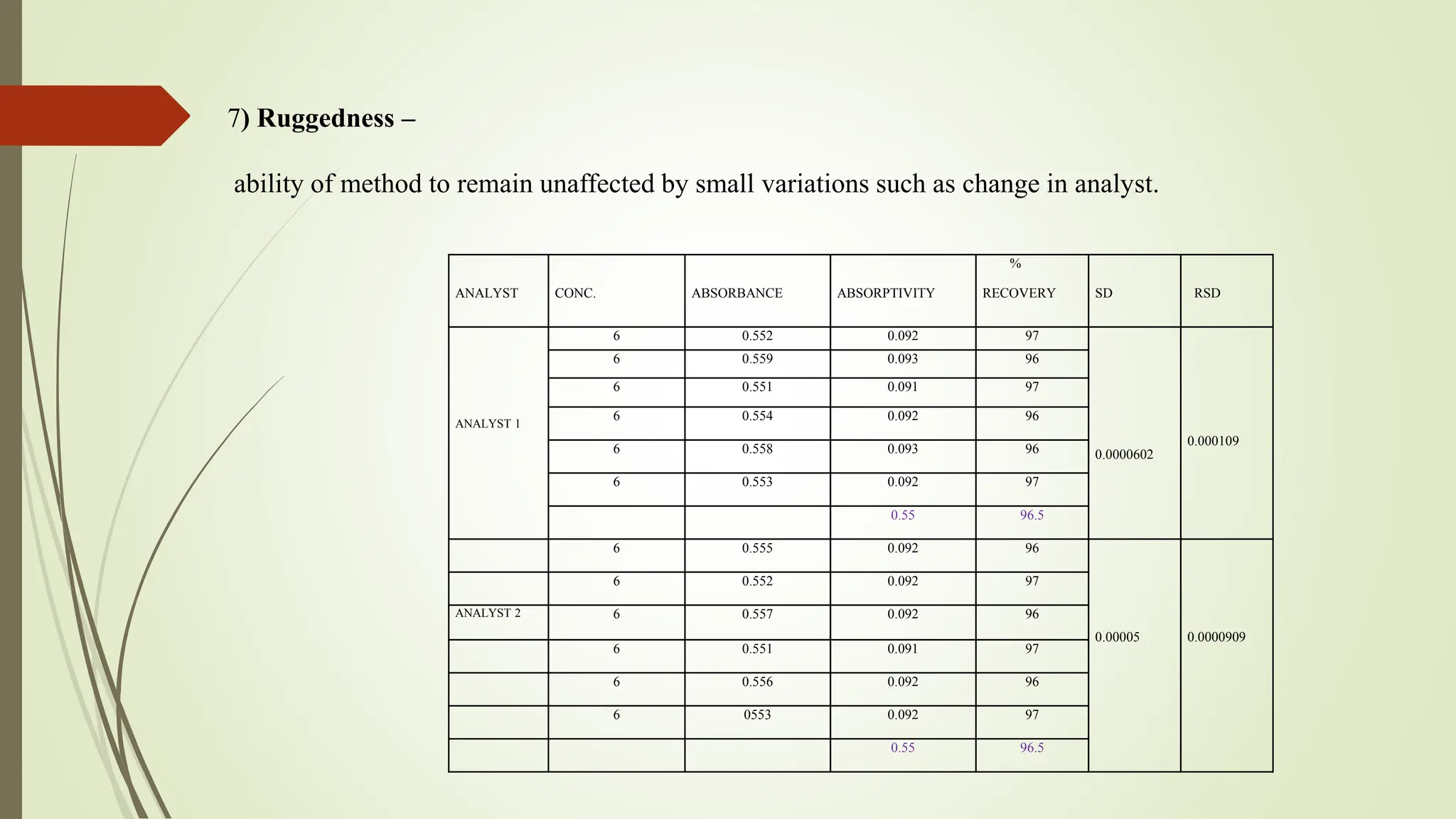
The document discusses the analytical method development and validation for tizanidine hydrochloride using UV spectroscopy. It outlines the aims, literature review, method development, optimization, validation studies, and future scope for improving the method. Key findings include the established linearity range of 2-12 μg/ml and correlation coefficient of 0.99961, validating the method's accuracy and precision.