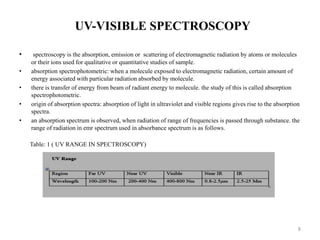
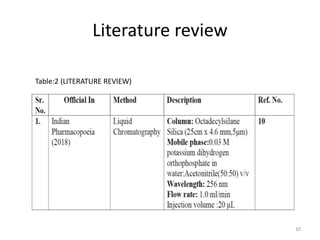
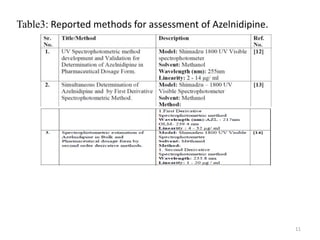
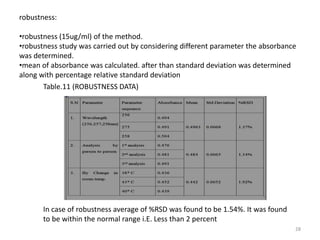
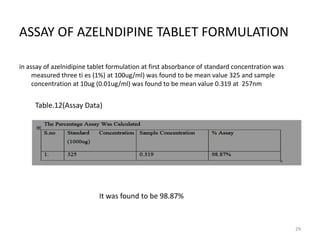
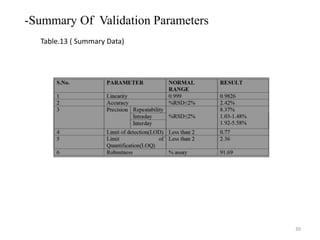
This document describes the development and validation of an analytical method for Azelnidipine using UV-Visible spectroscopy. The method development involved preparation of standard stock and working solutions, selection of the analytical wavelength of 257nm from UV scanning, and generation of a calibration curve. The method validation assessed parameters such as linearity, precision, accuracy, limit of detection, limit of quantification, robustness, and assay of Azelnidipine tablets to prove that the method is suitable for use in pharmaceutical analysis. The results of the study confirmed that UV-Visible spectroscopy can accurately and reliably measure Azelnidipine concentrations in formulations.
















![REFERENCES
• 1. koike h, kimura t, kawasaki t, et al. azelnidipine, a long-acting calcium channel blocker with slow onset and high vascular affinity [j].
annu rep sankyo res lab, 2002; 54:1-64
• 2. an, huamin, wang chencai., determination of content and related substance of azelnidipine by hplc, huaxi yaoxue zazhi, 2006; 21(6):
581-582.
• 3. ding, li, li, ma pengcheng, determination of azelnidipine in muman plasma by liquid chromatography-electron ionization- mass
spectroscopy, journal of pharmaceutical and biomedical analysis, 2007; 43(2): 575-579.
• 4. rele r.v., patil s.p... ultra-violet spectrophotometric method for estimation of azelnidipine from bulk drug and pharmaceutical
formulation. asian j. research chem. 3(4): oct. - dec. 2010; page 1077-1079.
• 5. kunti d. raskapur*, mrunali m. patel, anandkumari d. captain quality assurance, a. r. college of pharmacy, vallabh vidhyanagar,
gujarat, india,department of pharmaceutical chemistry, a. r. college of pharmacy, vallabh vidhyanagar, gujarat, india. email:
kunti_9@yahoo.com (received: 20 aug 2011, revised and accepted: 28 sep 2011)
• 6. spectrophotometric estimation of azelnidipine in bulk and pharmaceutical dosage form by second order derivative methods rele
rajan v.central research laboratory, d. g. ruparel college, matunga, mumbai available online journal of chemical and pharmaceutical
research, 2014, 6(8):198-202
• 7.m.s. charde, a.s. welankiwar, j. kumar, method development by liquid chromatography with validation, international journal of
pharmaceutical chemistry, 4 (1) (2014) 57-61.
• 8. n. n. godswill, n. e. g. frank, m. y. j. edson, y. emmanue, b. j. martin, n.b. hermine, t. m. kingsley, l. l.n. b. constant, n. m. armand,
gc- fid method development and validation parameters for analysis of palm oil (elaeis guineensis jacq.) fatty acids composition, research
in plant sciences, 2 (3) (2014), 53
33](https://image.slidesharecdn.com/analyticalmethoddevelopmentandvalidation-210812142708/85/Analytical-method-development-and-validation-33-320.jpg)