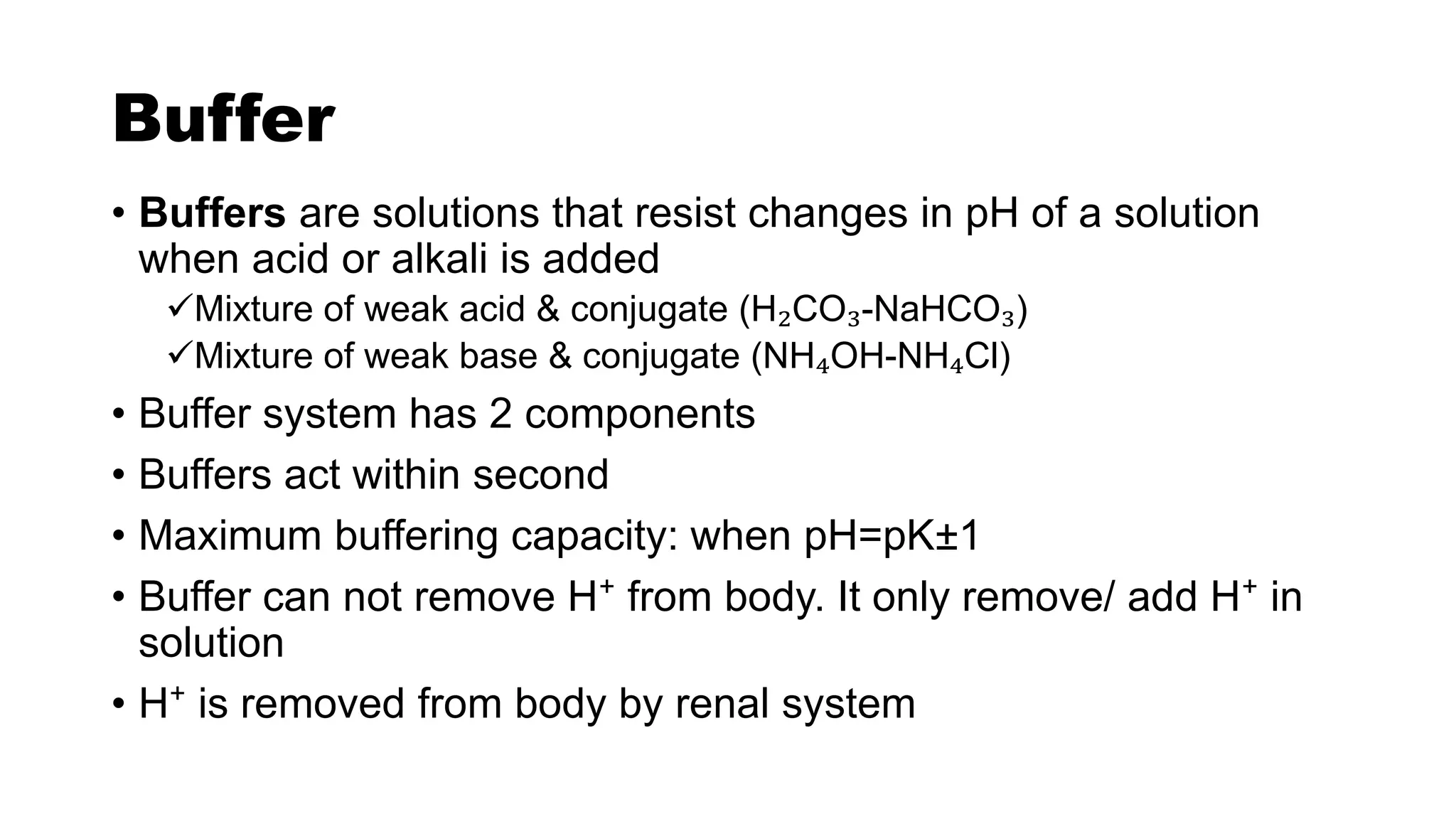
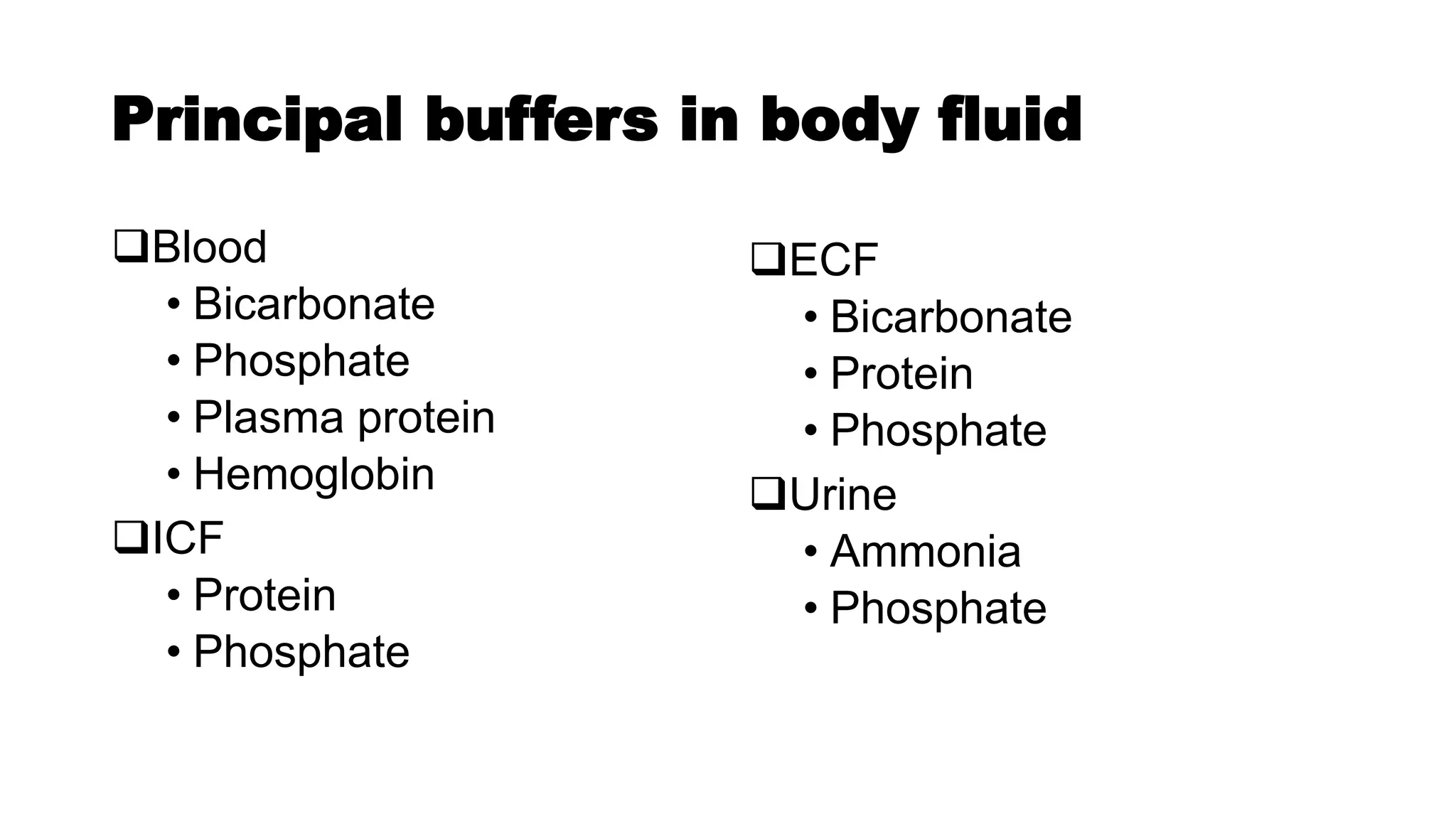
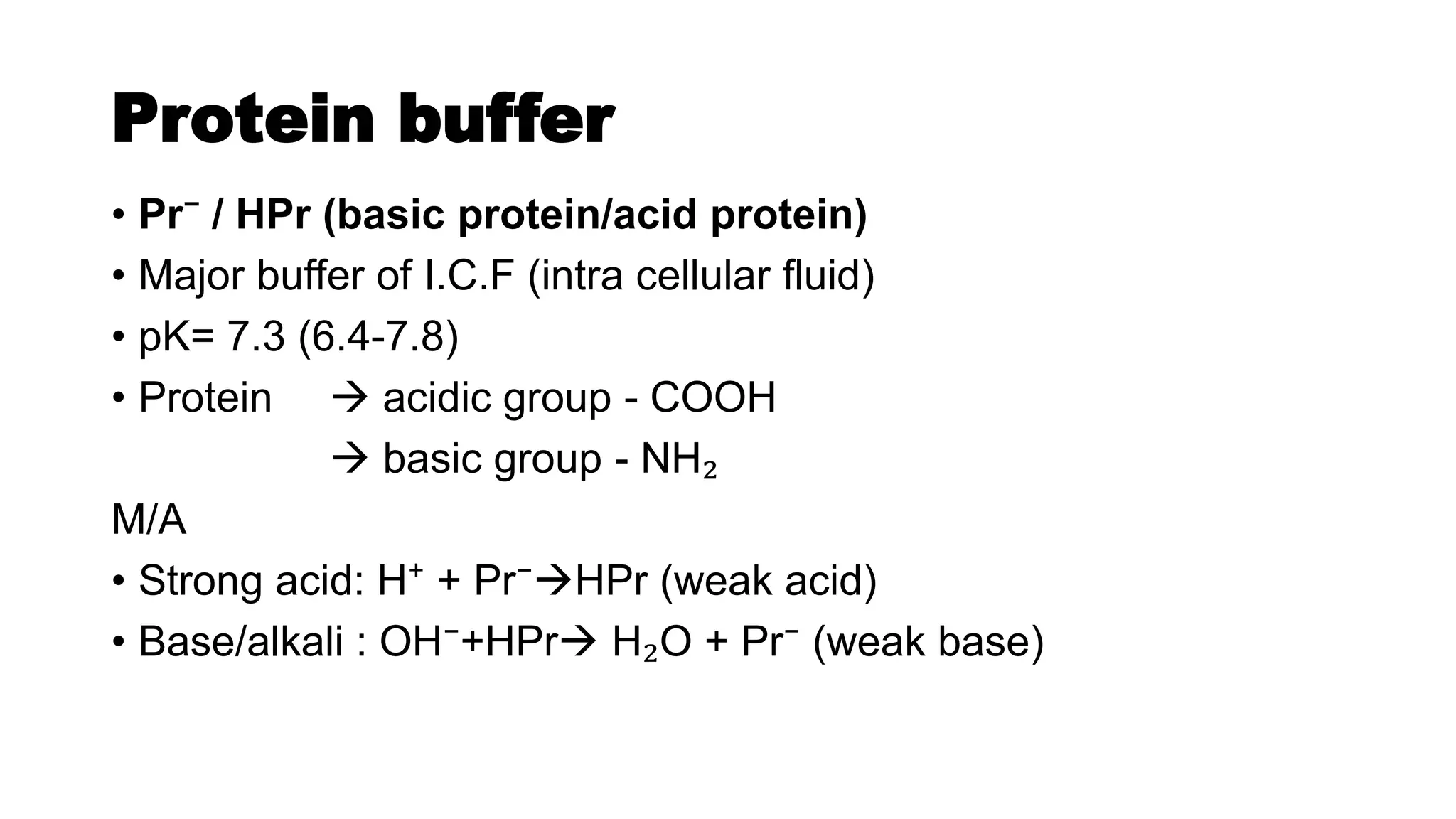
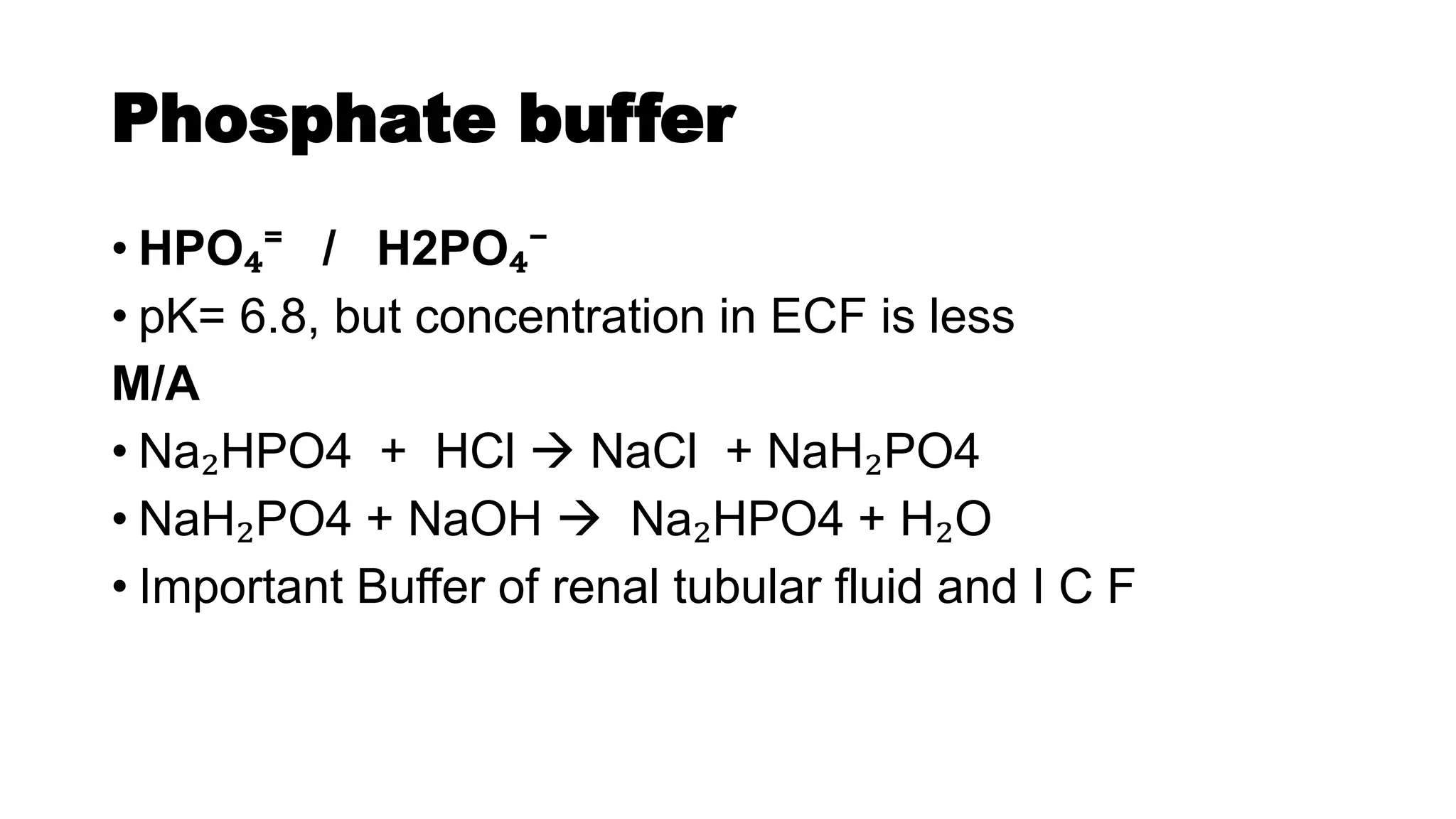
The document discusses acid-base chemistry, defining acids as proton donors and bases as proton acceptors, alongside their properties and examples. It explains the concept of pH, its measurement, and the significance of buffers in maintaining pH levels in biological systems, particularly highlighting the bicarbonate buffer system. Additionally, the document includes the Henderson-Hasselbalch equation, which is crucial for understanding buffer action and acid-base balance.


![Acid
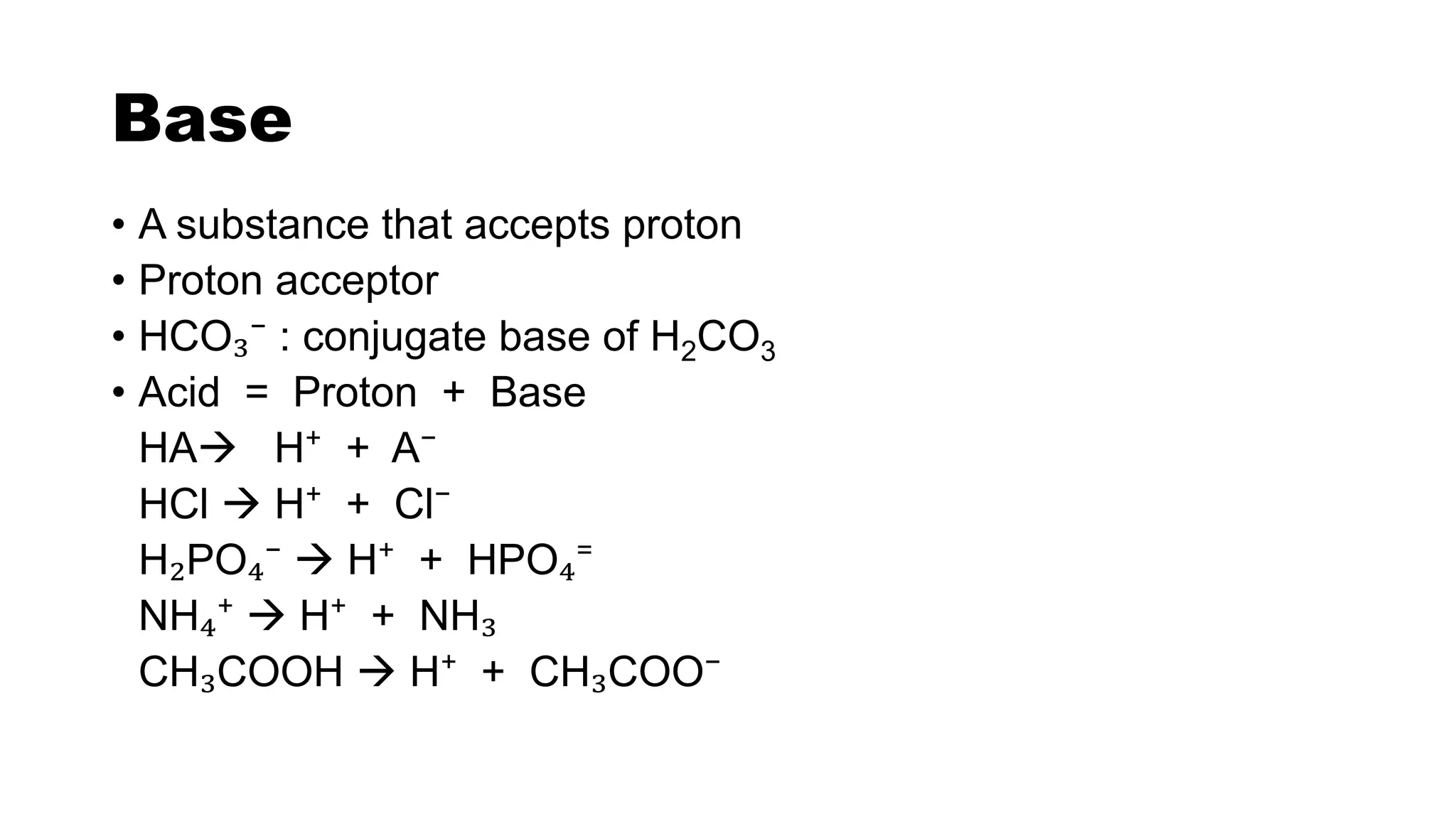
• A substance that gives off proton [H⁺]
• Proton donor
• H2CO3 ↔ H⁺ + HCO₃⁻
Properties-
Sour in taste
Convert blue litmus into red
Produce salt and water with alkali
Gives H⁺ in aqueous solution](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-3-2048.jpg)

![pH
• Introduced by Sorensen in 1909
• pH is defined as the negative log of the hydrogen ion
concentration.
The equation is:
pH = - log [H+]
• Pure water has an equal concentration of H+ & OH⁻ ions
[H+] = [HO⁻]= 10⁻⁷ mole/L
So, pH= 7 which is neutral.
• pH of blood plasma 7.35-7.45
• pH of gastric juice 1.5-3
• pH of urine 4.5-6.8](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-6-2048.jpg)
![pH scale
• The pH scale measures how
acidic or basic a substance
is.
• Range : 0 to 14.
pH 7 = neutral.
pH < 7 = acidic.
pH > 7 = basic.
pH = 0, [H⁺] is maximum
• The pH scale is logarithmic
• pH 4
• 10 times more acidic than
pH 5
• 100 times (10 x 10) more
acidic than pH 6.
• pH 10
• 10 times more alkaline
than pH 9
• 100 times (10 x 10) more
alkaline than pH 8](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-7-2048.jpg)
![[H⁺] of blood = 40 nmol/L](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-8-2048.jpg)


![pK
• Acid dissociates as,
HA H⁺ + A⁻
• By the law of mass action: The product of the concentration of
products in a chemical reaction divided by the product of the
concentration of the reactants at equilibrium is a constant
K=
𝐻⁺ [𝐴⁻ ]
[𝐻𝐴]
• Relative strength of weak acid & base is expressed in terms of
their ‘K’
• For strong acid, K= high
• For weak acid, K= low](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-11-2048.jpg)
![Henderson-Hasselbalch equation
• Important for understanding buffer action and acid-base
balance
• For the dissociation of a weak acid HA into H+ and A-, the
Henderson-Hasselbalch equation can be derived as follows:
• HA dissociates as follows
HA ↔ H⁺ + A⁻
• The equilibrium constant for this dissociation is-
K=
𝐻⁺ [𝐴⁻ ]
[𝐻𝐴]](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-12-2048.jpg)
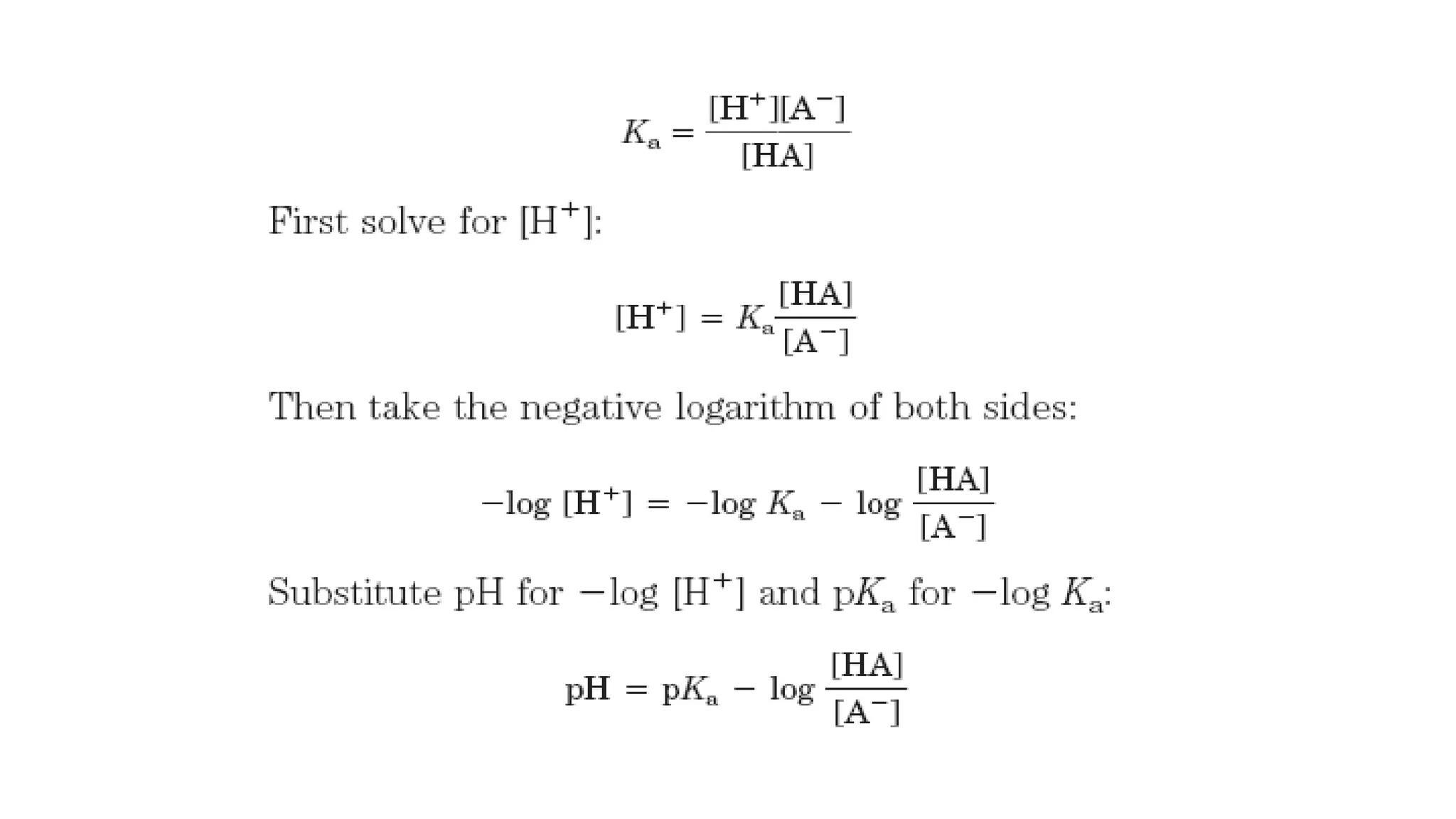
![Henderson-Hasselbalch equation
• Now invert -log [HA]/[A-], which involves changing its
sign, to obtain the Henderson-Hasselbalch equation:
• It shows the pK of a weak acid is equal to the pH of the
solution when [HA] = [A-] (i.e. the acid is exactly half
neutralized)](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-14-2048.jpg)
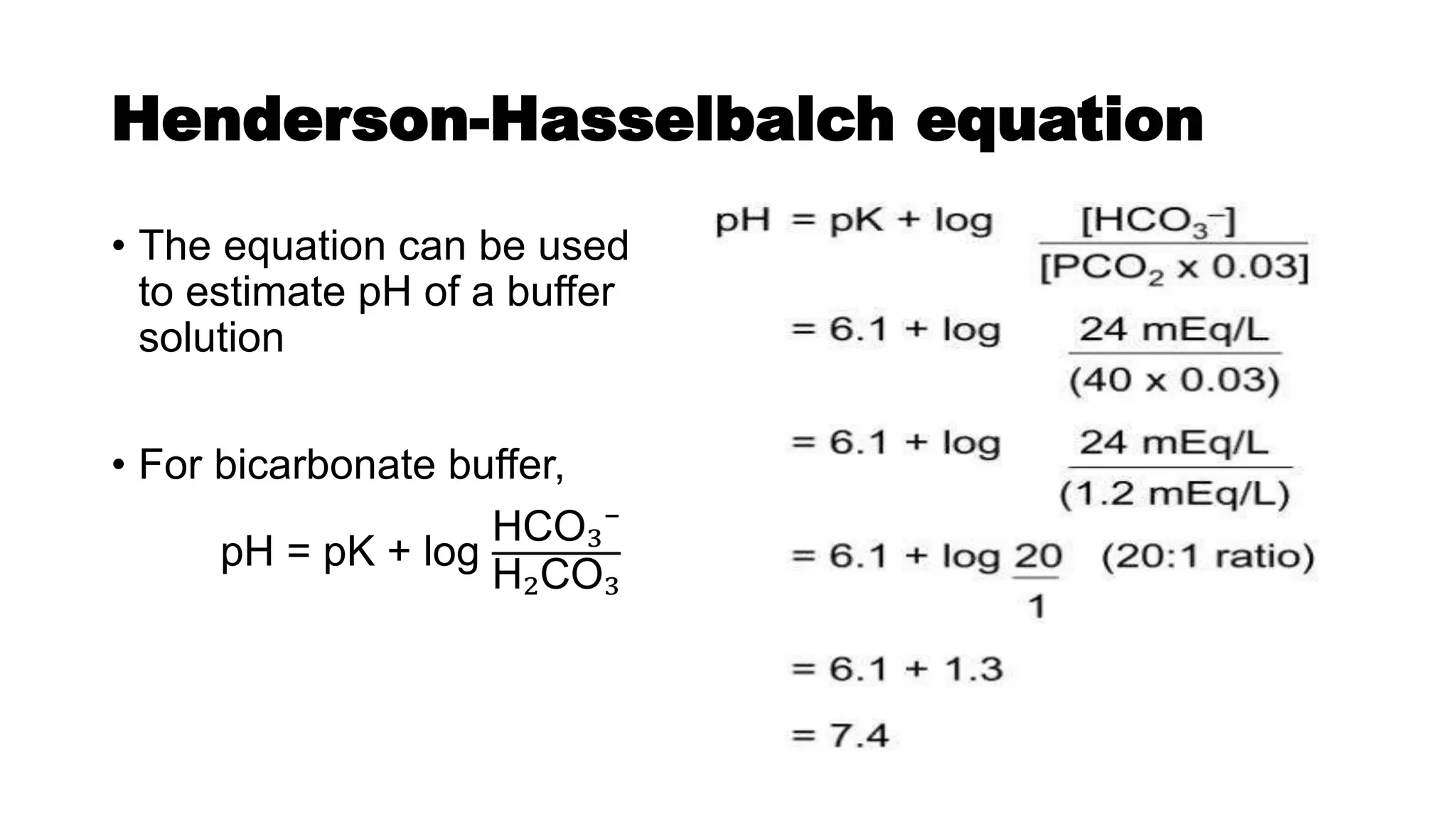
![Regulation of [H⁺]
• Acids and bases continually enter and exit body.
• For normal function of body and normal enzyme activity a
normal [H⁺] (normal pH) is essential.](https://image.slidesharecdn.com/acidbasebuffer-210120122439/75/Acid-Base-pH-Buffer-16-2048.jpg)