The document discusses acid-base balance and summarizes key concepts from traditional and modern physical-chemical approaches. It explains that the traditional view focused on hydrogen and bicarbonate ion concentrations, while the Stewart model emphasizes three independent variables: partial pressure of carbon dioxide, non-volatile weak acid concentration, and strong ion difference. The Stewart approach provides a more comprehensive understanding of factors influencing pH.


![•The acidity of the solution is thus a measure of the hydrogen
ion activity
•The normal concentration of H+ is in nanomole range (nmol/L)
• 1 nmol/L = 10 -6
milliequivalents/L
•Serum sodium concentration is 3 million times H+
concentration
•Becoz such figures and units may be confusing, H+
concentration expressed as pH units
•pH = negative log10 of hydrogen ion concentration in nmol/l
•The p refers to the German word ‘potenz’ (power) so pH
means 'power of hydrogen'.
pH = -log10[H+]](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-5-320.jpg)
![Relationship between pH and
[H+
]
pH
[H+
]
(nanomoles/l)
6.8 158
6.9 125
7.0 100
7.1 79
7.2 63
7.3 50
7.4 40
7.5 31
7.6 25
7.7 20
7.8 15
A doubling or a halving of
[H+] means a change in
pH by 0.3 either up or
down.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-6-320.jpg)
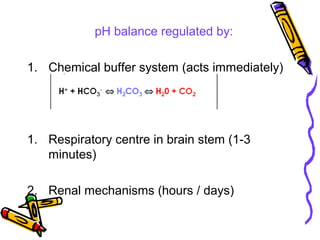

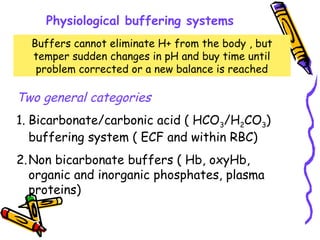
![• pH = pKa + log [HCO3
-
]/[H2CO3]
• pH = pKa + log [HCO3
-
]/0.03 x PCO2
• pH = 6.1 + log [HCO3
-
]/0.03 x PCO2
• 7.4 = 6.1 + log 20/1
• 7.4 = 6.1 + 1.3
• The solubility constant of CO2 is 0.03
• The pKa of carbonic acid is 6.1
• Plasma pH equals 7.4 when buffer ratio is 20/1
• Plasma pH may be affected by a change in either the
bicarbonate concentration or the PCO2
• The [HCO -
] and PCO values determine plasma pH
Henderson-Hasselbalch equation
Expresses the relationship of the HCO3/H2CO3
buffering system to pH](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-14-320.jpg)
![• pH = pKa + log [HCO3
-
]/[H2CO3]
• pH = pKa + log [HCO3
-
]/0.03 x PCO2
• pH = 6.1 + log [HCO3
-
]/0.03 x PCO2
• Disadvantages of HH
• Better quantification of resp component than metabolic
• No quantification of non carbonic acids
Henderson-Hasselbalch equation
Expresses the relationship of the HCO3/H2CO3
buffering system to pH](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-15-320.jpg)
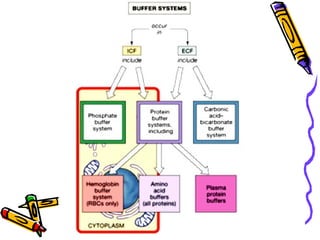
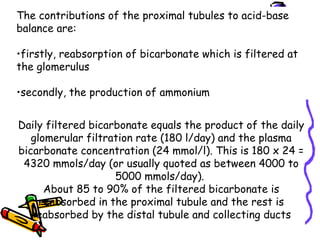
![Renal Mechanisms
• Kidneys alter/replenish H+
by altering
plasma [HCO3
-
]
∀↓ [H+
] plasma (alkalosis) → kidneys
excrete lots of HCO3
-
∀↑ [H+
] plasma (acidosis) → kidneys
produce new HCO3
-](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-21-320.jpg)

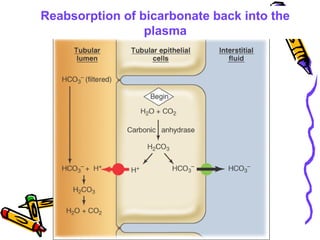


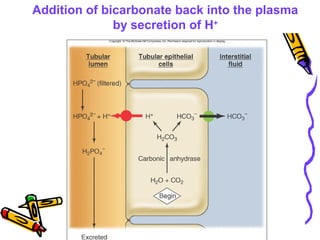
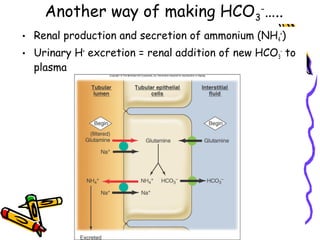
![Renal responses to acidosis
• H+
ions secreted to reabsorb all filtered HCO3
-
• Even more H+
secreted, contributing new HCO3
-
to
plasma as these H+
ions are excreted bound to
non-HCO3
-
urinary buffers such as HPO4
2-
• Tubular glutamine metabolism and ammonium
excretion are enhanced to make more HCO3
-
(TAKES TIME!!!)
• NET RESULT: More new HCO3
-
into blood,
increasing plasma [HCO3
-
]. This compensates for
the acidosis. Urine is highly acidic (lowest pH is
4.4)](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-29-320.jpg)

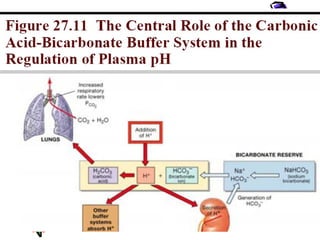
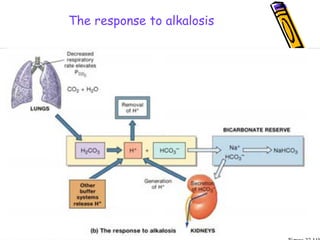
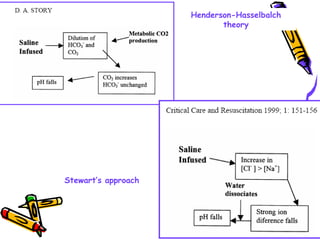
![Traditional view
When we first study acid-base balance, it is too easy
believe that the concentrations of the hydrogen and
bicarbonate ions, [H+] and [HCO3-], are at the heart
of the problem - are dominant forces.
We do, after all, discuss them, measure them, and
treat them:
Whatever an acid or a base does, must be due to the
pH, i.e., the concentration of H+.
In addition [HCO3-] must surely determine the
metabolic state.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-34-320.jpg)

![Stewart's Independent Variables:
There are three variables which are
amenable to change in-vivo:
1. partial pressure of carbon dioxide (PCO2),
2. total weak non-volatile acids [ATOT],
3.net Strong Ion Difference [SID].
The influence of these three variables can
be predicted through six simultaneous
equations](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-37-320.jpg)
![Stewart's Dependent Variables:
Stewart listed a total of six ion
concentrations as dependent:
[H+], [OH-], [HCO3-], [CO3--2], [HA], [A-]
(weak acids and ions).
In-vivo and clinically, therefore, these are
not subject to independent alteration.
Their concentrations are governed by
concentrations of other ions and
molecules.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-38-320.jpg)
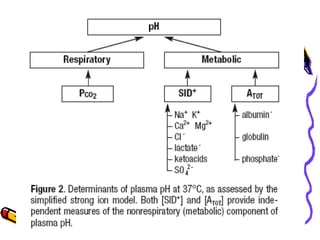
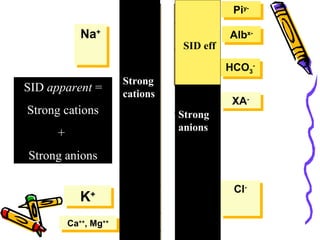
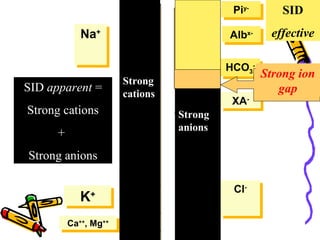
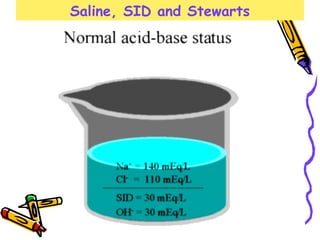
![[SID]:
The Strong Ion Difference is the difference
between the sums of concentrations of the strong
cations and strong ions:
[SID] = [Na+] + [K+] + [Ca2+] + [MG2+] - [CL-]
– [Other Strong Anions].](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-42-320.jpg)
![[ATOT]:
[ATOT] is the total plasma concentration of
the weak non-volatile acids, inorganic phosphate,
serum proteins, and albumin:
[ATOT] = [PiTOT] + [PrTOT] + albumin.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-43-320.jpg)
![Total CO2:
Predominantly pCO2, also H2CO3, carbonates
The effects of changes on PCO2 are well understood
and produce the expected alterations in [H+]:
CO2 + H2O <—> H2CO3 <—> HCO3- + H+](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-44-320.jpg)
![Metabolic (Non-Respiratory):
Metabolic disturbances, obviously, cannot be viewed as a
consequence of bicarbonate concentration because
bicarbonate is merely a dependent variable.
The two possible sources of metabolic disturbances are either
[SID] or [ATOT].
With normal protein levels, [SID] is about 40mEq/L
Any departure from this normal value is roughly equivalent to
the standard base excess (SBE), i.e., if the measured [SID]
were 45 mEq/L, the BE would be about 5 mEq/L, and a
measured [SID] of 32 mEq/L would approximate to a BE = -8
mEq/L.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-45-320.jpg)
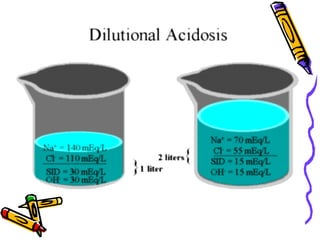
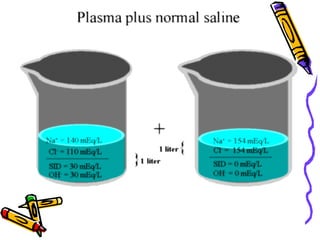
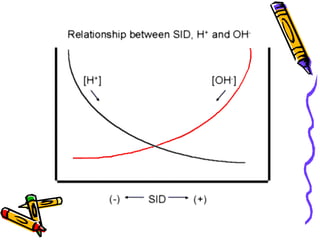
![Changing [SID]:
[SID] can be changed by two principal methods:
1) Concentration:
•Dehydration or over-hydration alters the concentration
of the strong ions and therefore increases, or decreases,
any difference.
•The body's normal state is on the alkaline side of neutral.
•Therefore, dehydration concentrates the alkalinity
(contraction alkalosis) and increases [SID];
•Overhydration dilutes this alkaline state towards neutral
(dilutional acidosis) and decreases [SID].
2) Strong Ion Changes:
If the sodium concentration is normal, alterations in the
concentration of other strong ions will affect [SID]:](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-46-320.jpg)
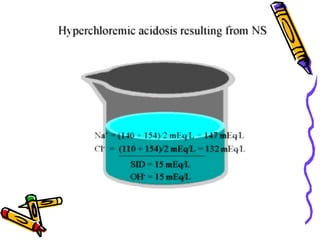
![2) Strong Ion Changes:
If the sodium concentration is normal, alterations in the
concentration of other strong ions will affect [SID]:
a. Inorganic Acids:
The only strong ion capable of sufficient change is
chloride, Cl- (potassium, calcium and magnesium do
not change significantly). An increased Cl- concentration
causes an acidosis and a decreased [SID] causes
alkalosis.
Because the chloride ions are measured, the anion gap
will be normal.
b. Organic Acids:
By contrast, if the body accumulates one of the organic
acids, e.g., lactate, formate, keto-acids, then the
metabolic acidosis is characterized by a normal chloride
concentration and an abnormal anion gap because of the
presence of the "unmeasured" organic acid.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-47-320.jpg)
![Changing [ATOT]:
The non-volatile weak acids comprise inorganic phosphate,
albumin and other plasma proteins. Making the greatest
contribution to acid-base balance are the proteins, particularly
albumin, which behave collectively as a weak acid.
Hypoproteinemia, therefore, causes a base excess and vice
versa.
Phosphate levels are normally so low that a significant fall is
impossible. However, in renal failure, high phosphate levels
contribute to the acidemia.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-49-320.jpg)
![Pros and Cons:
1) Understanding:
Stewart's greatest contribution may be his focus on the
importance of the factors controlling pH.
[H+], [OH-] and [HCO3-] are merely dependent
variable.
This emphasis on the importance of the underlying
causes rightly diminishes the importance of the
bicarbonate ion.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-50-320.jpg)
![2) Shortcomings :
A major shortcoming lies in calculating a value for
[SID] which depends upon accurate measurements of
several variables.
An acceptable level of error in the underlying
measurements becomes less acceptable after
subtraction.
This is partly because the errors are summed and
partly because any error now appears proportionately
large against the resulting small value.](https://image.slidesharecdn.com/07-140422135054-phpapp02/85/07-acid-base-disorders-51-320.jpg)