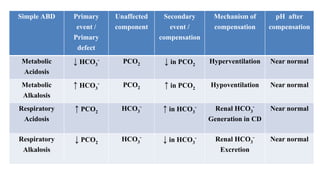
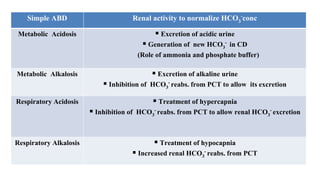
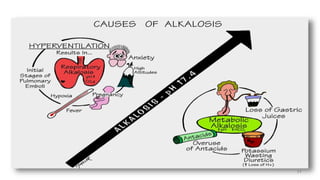
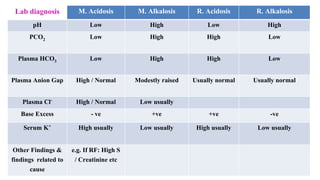
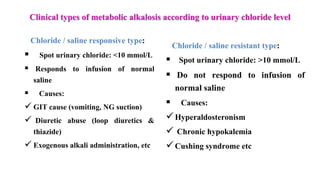
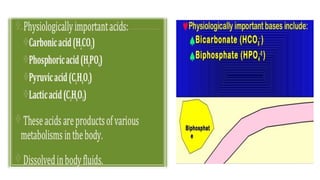
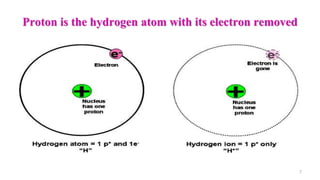
The document provides a comprehensive overview of acid-base balance, including definitions, types of acids and bases, their sources, and the significance of pH in biological systems. It discusses acid-base disorders, compensation mechanisms, and methods for assessing and correcting these imbalances. Additionally, it covers the importance of buffers, particularly the bicarbonate buffer system, in maintaining normal body pH and the associated physiological implications of deviations from normal pH levels.

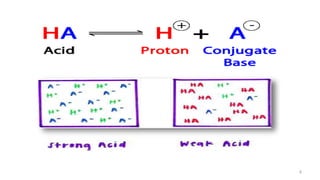
![ Proton (H
+
) donor in aqueous
solution
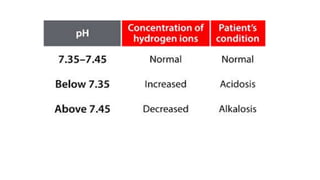
pH <7
Produces H+ when dissolved in water
May be charged particle [NH4
+,
H2PO4
-] or may be without charge
[HCl]
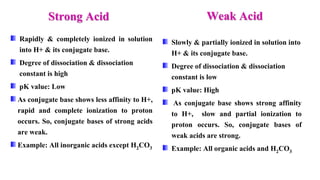
Types: Strong acid , Weak acid
Proton (H+) acceptor in aqueous
solution
pH >7
Produces OH- when dissolved in water
May be charged particle [Cl-, HCO3
-]
or may be without charge [NH3]
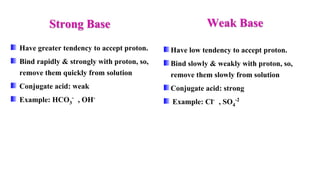
Types: Strong base , Weak base
Acid Base](https://image.slidesharecdn.com/acidbasebalanceabdformdms-201015070317/85/Acid-base-balance-updated-in-2020-3-320.jpg)










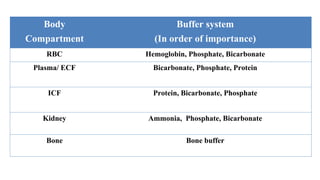
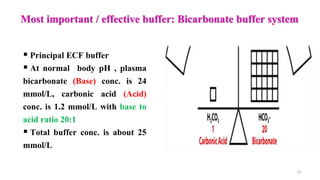
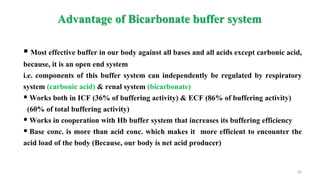








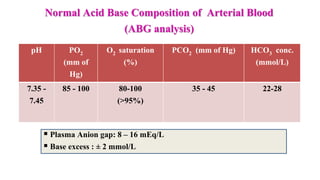

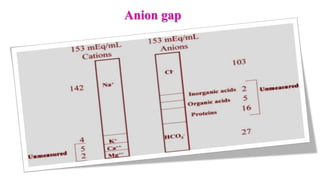
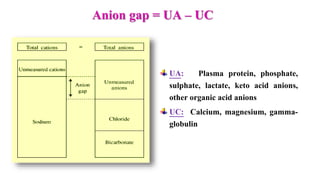
![ Normal range: 8 – 16 mEq/L
[K level is ignored since plasma K+
concentration doesn’t vary that
much]
Importance of AG:
It helps to differentiate the causes
of metabolic acidosis
It helps to determine the nature of
metabolic acidosis (simple /
complex)](https://image.slidesharecdn.com/acidbasebalanceabdformdms-201015070317/85/Acid-base-balance-updated-in-2020-56-320.jpg)
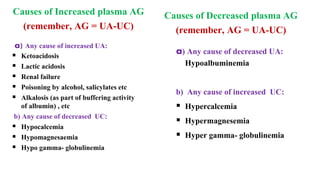
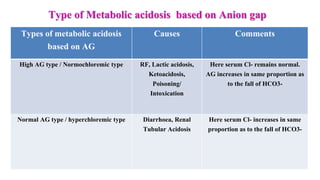
![Base Excess
Difference between present /actual
bicarbonate concentration of an individual
and the standard bicarbonate concentration
Base Excess = [HCO3
-] p – [HCO3
-]std
Positive BE: High plasma HCO3
- conc. which
is found in metabolic alkalosis & respiratory
acidosis
Negative BE: Low plasma HCO3
- conc. which
is found in metabolic acidosis & respiratory
alkalosis](https://image.slidesharecdn.com/acidbasebalanceabdformdms-201015070317/85/Acid-base-balance-updated-in-2020-59-320.jpg)
![Standard plasma bicarbonate concentration:
It is the plasma bicarbonate concentration of an individual of an
individual at:
Body temperature 37 degree C
Arterial PCO2 at 40 mm Hg
Hb conc. normal 14-16 g/dl
Oxygen saturation of Hb normal >95%
Symbolized as [HCO3
-]std
24 mmol / L](https://image.slidesharecdn.com/acidbasebalanceabdformdms-201015070317/85/Acid-base-balance-updated-in-2020-60-320.jpg)