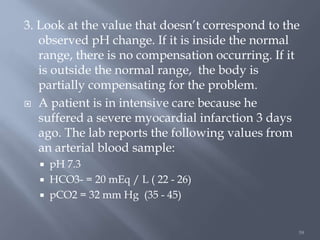
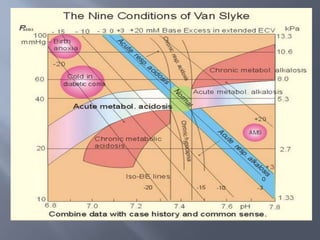
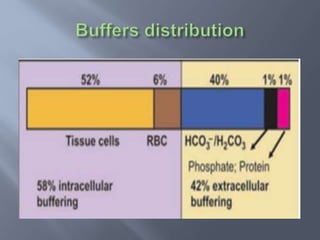
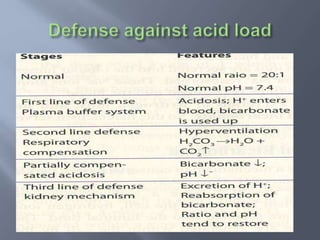
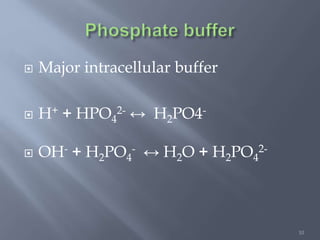
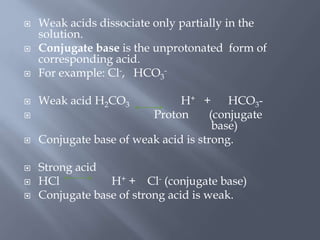
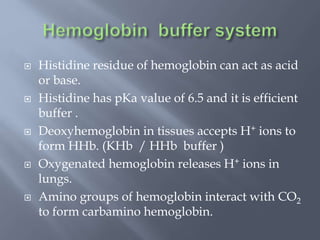
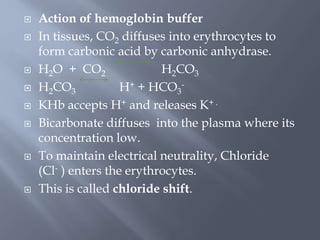
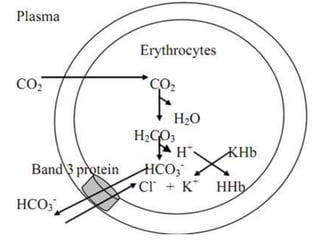
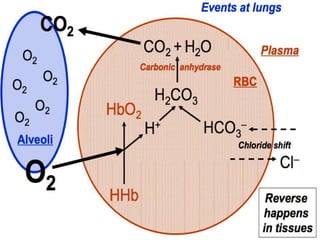
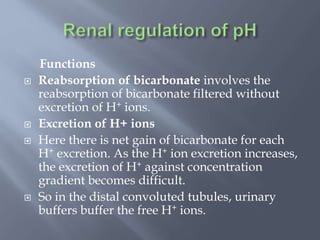
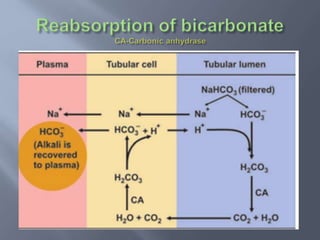
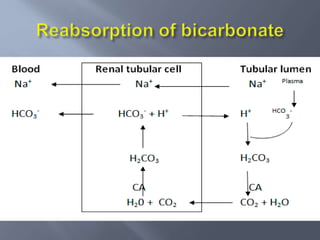
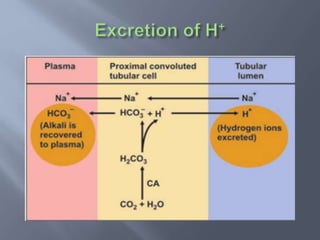
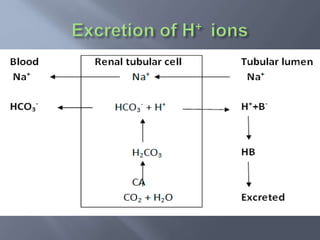
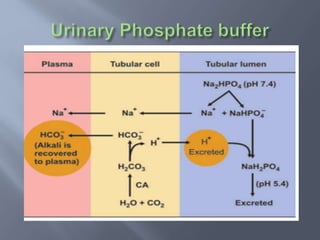
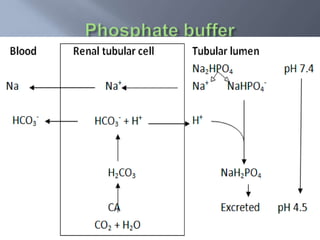
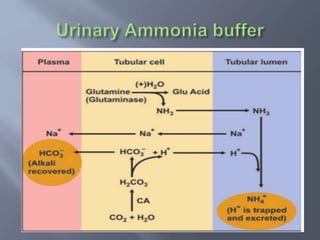
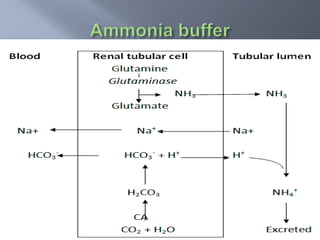
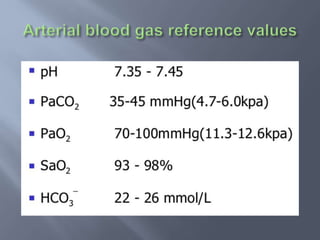
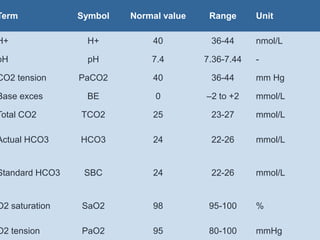
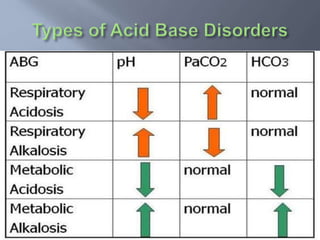
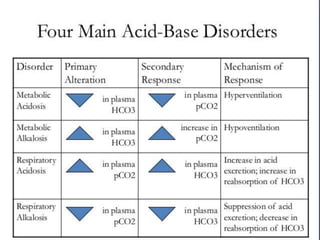
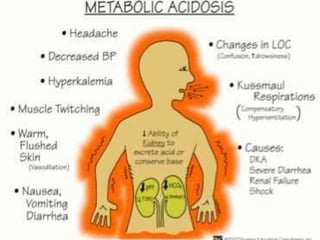
The document discusses the importance of metabolism and its relation to acid-base balance in the human body, highlighting the role of enzymes and the influence of pH. It explains the mechanisms of buffering, the effects of acidosis and alkalosis, and the regulatory functions of the kidneys and lungs in maintaining homeostasis. Additionally, it details the physiological and clinical implications of varying pH and bicarbonate levels in different metabolic conditions.






























![57
Mechanism: Renal loss of bicarbonate causes a
further fall in plasma bicarbonate (in addition
to the acute drop due to the physicochemical
effect and protein buffering).
Magnitude: An average 5 mmol/l decrease in
[HCO3-] per 10mmHg decrease in pCO2 from
the reference value of 40mmHg. This maximal
response takes 2 to 3 days to reach.
Limit: The limit of compensation is a [HCO3-]
of 12 to 15 mmol/l.](https://image.slidesharecdn.com/acidbasedisorders-200520044331/85/Acid-base-disorders-57-320.jpg)