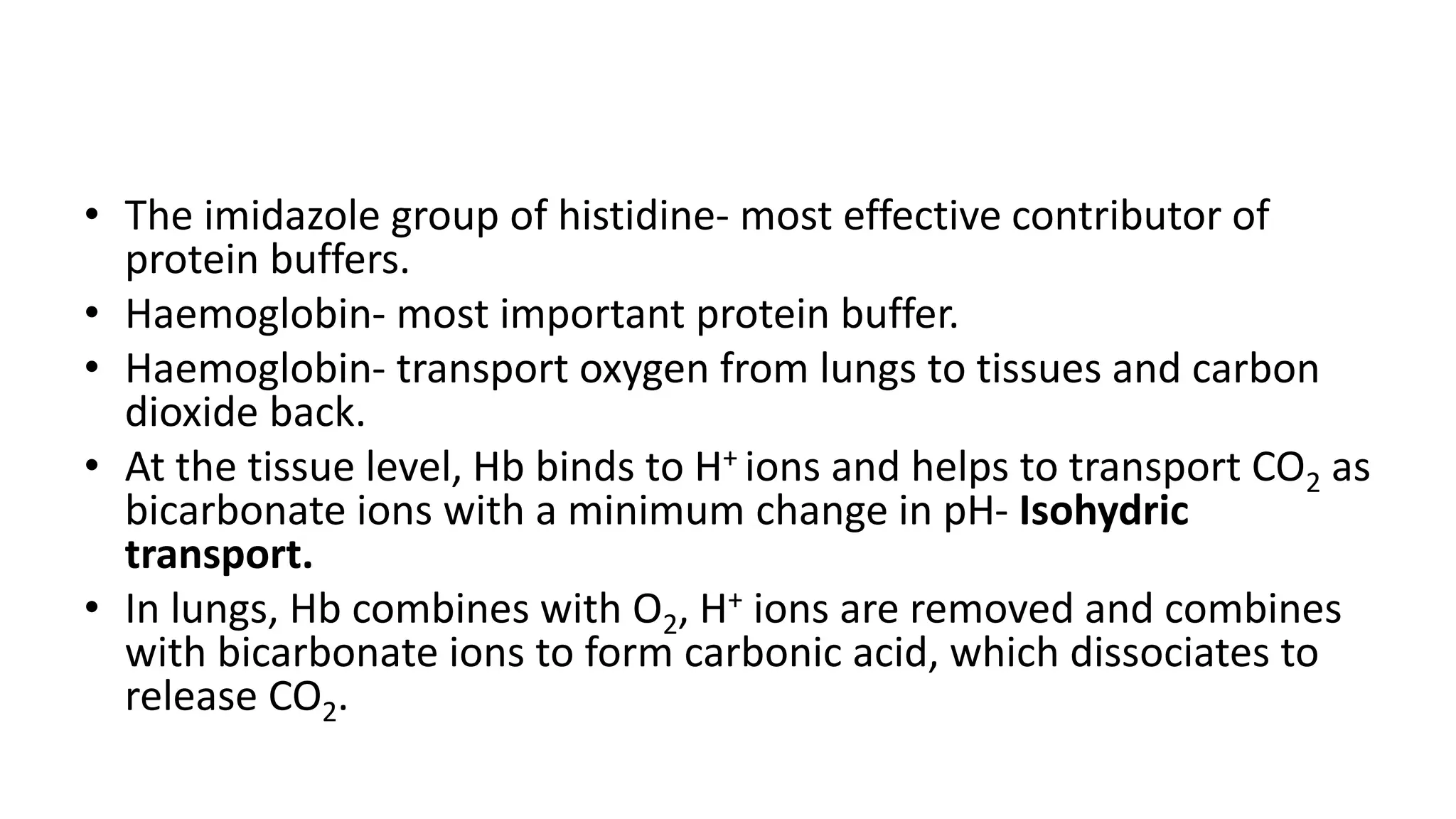
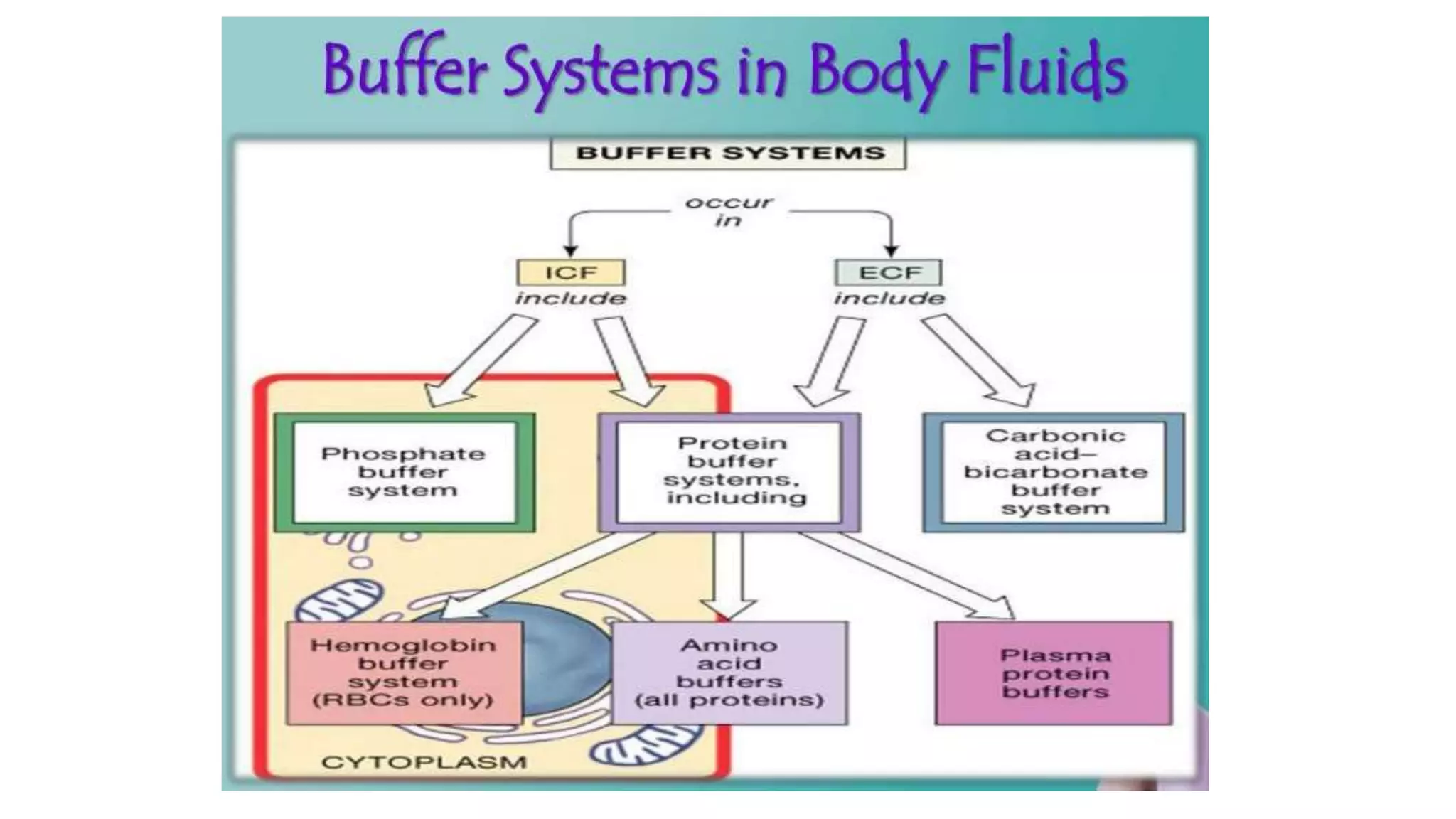
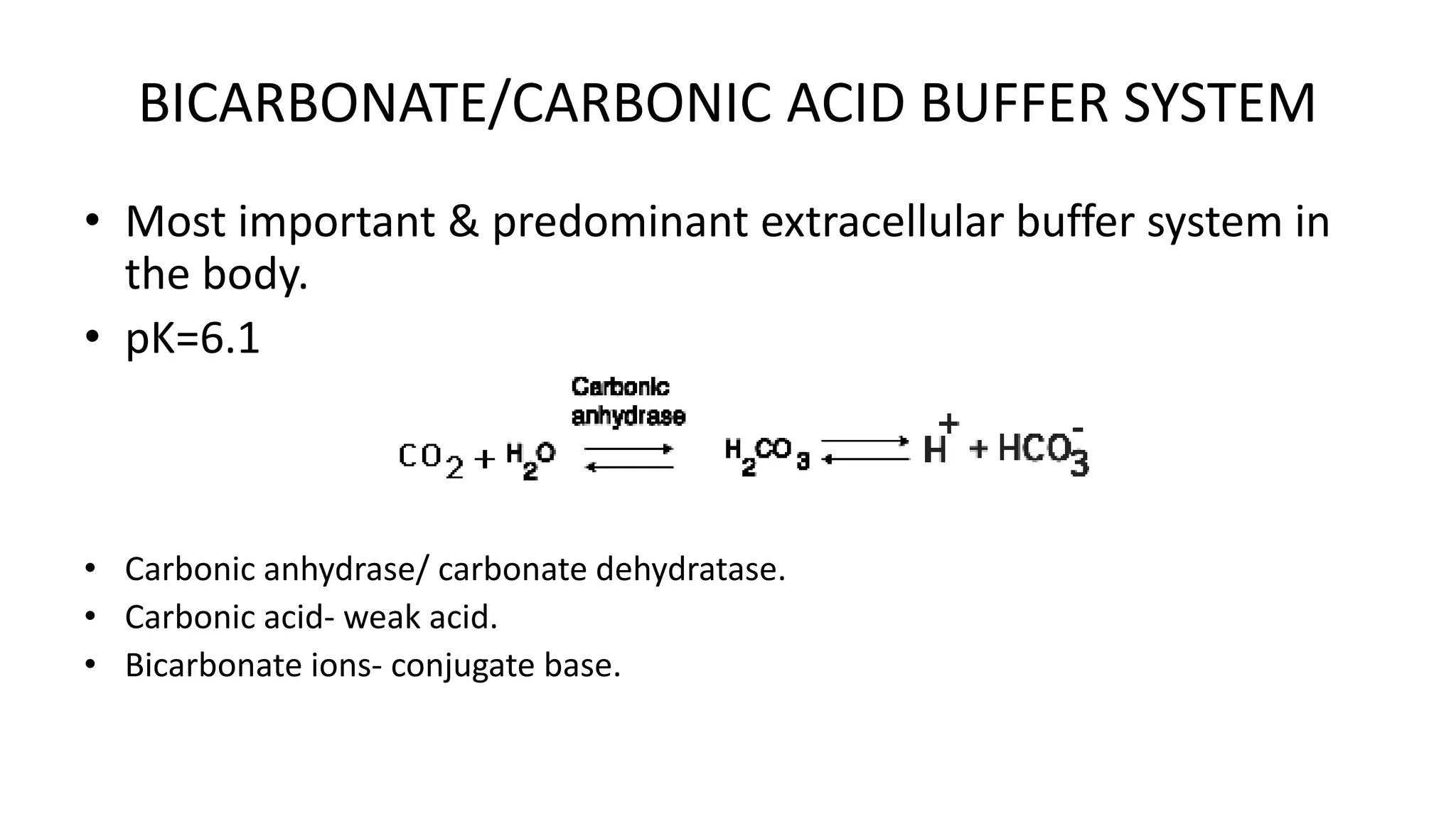
The document discusses physiological buffers, including acids, bases, and their importance in maintaining blood pH levels between 7.35-7.45. It explains the bicarbonate/carbonic acid buffer system and highlights the roles of protein and phosphate buffer systems in cellular processes. Additionally, it addresses conditions like acidosis and alkalosis, detailing their clinical causes and compensatory mechanisms in response to pH changes.










![Medical conditions associated with blood pH
ACIDOSIS
• Blood pH goes DOWN (becomes acidic)
• [H+] increases; pH decreases (pH <7.35)
• Renal compensation
• Two types:
Metabolic Acidosis- due to decrease in bicarbonate- faster respiration
Respiratory Acidosis- due to increase in carbonic acid- slower respiration
• [CO2] increases
• Equilibrium shifts to RIGHT](https://image.slidesharecdn.com/physiologicalbuffers-new-170331022722/75/Physiological-buffers-new-13-2048.jpg)

![ALKALOSIS
• Blood pH goes UP (becomes alkaline)
• [H+] decreases; pH increases (pH>7.45)
• Two types:
Respiratory Alkalosis- due to decrease in carbonic acid- faster respiration
Metabolic Alkalosis- due to increase in bicarbonate- slower respiration
• [CO2] decreases
• Equilibrium shifts to LEFT](https://image.slidesharecdn.com/physiologicalbuffers-new-170331022722/75/Physiological-buffers-new-15-2048.jpg)