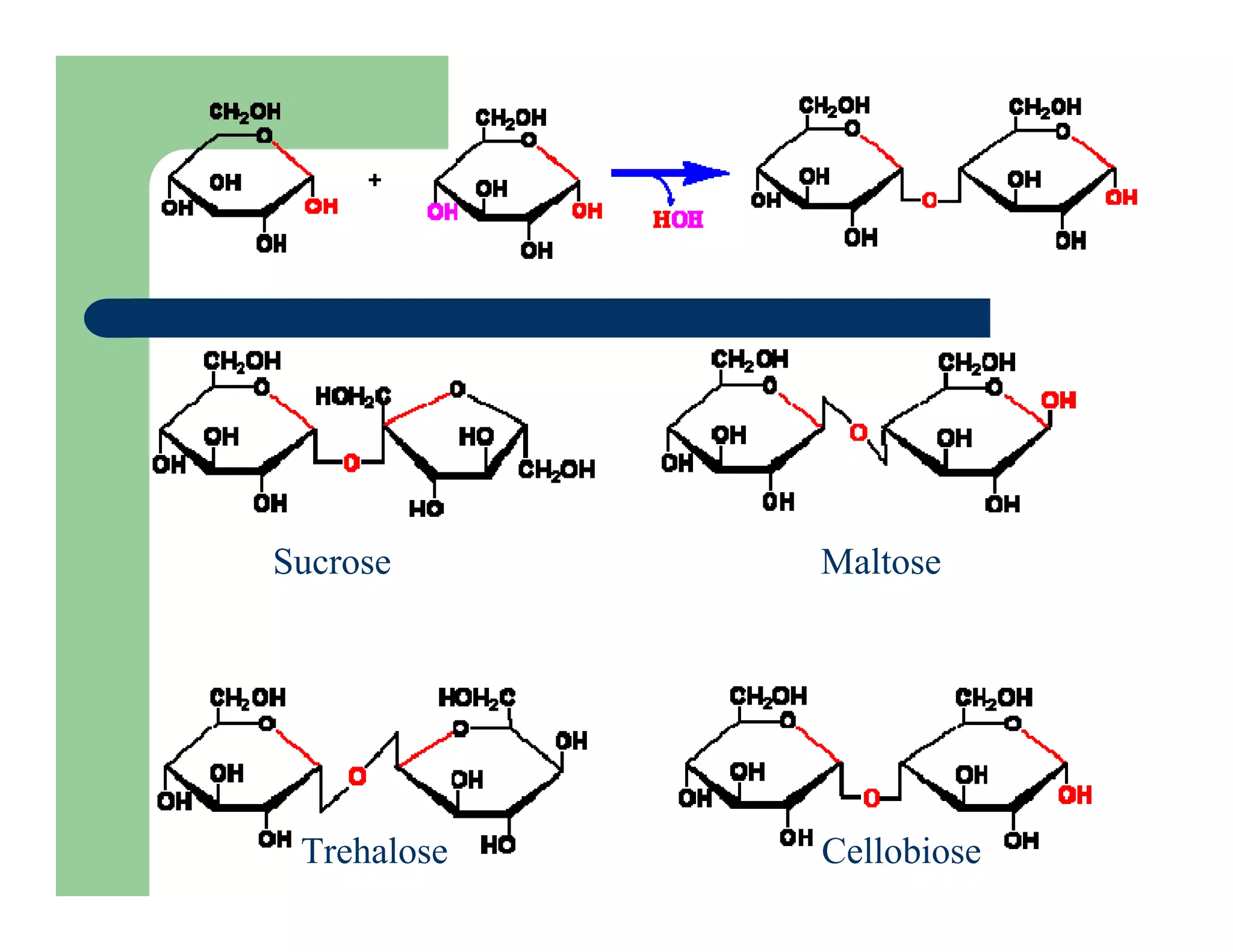
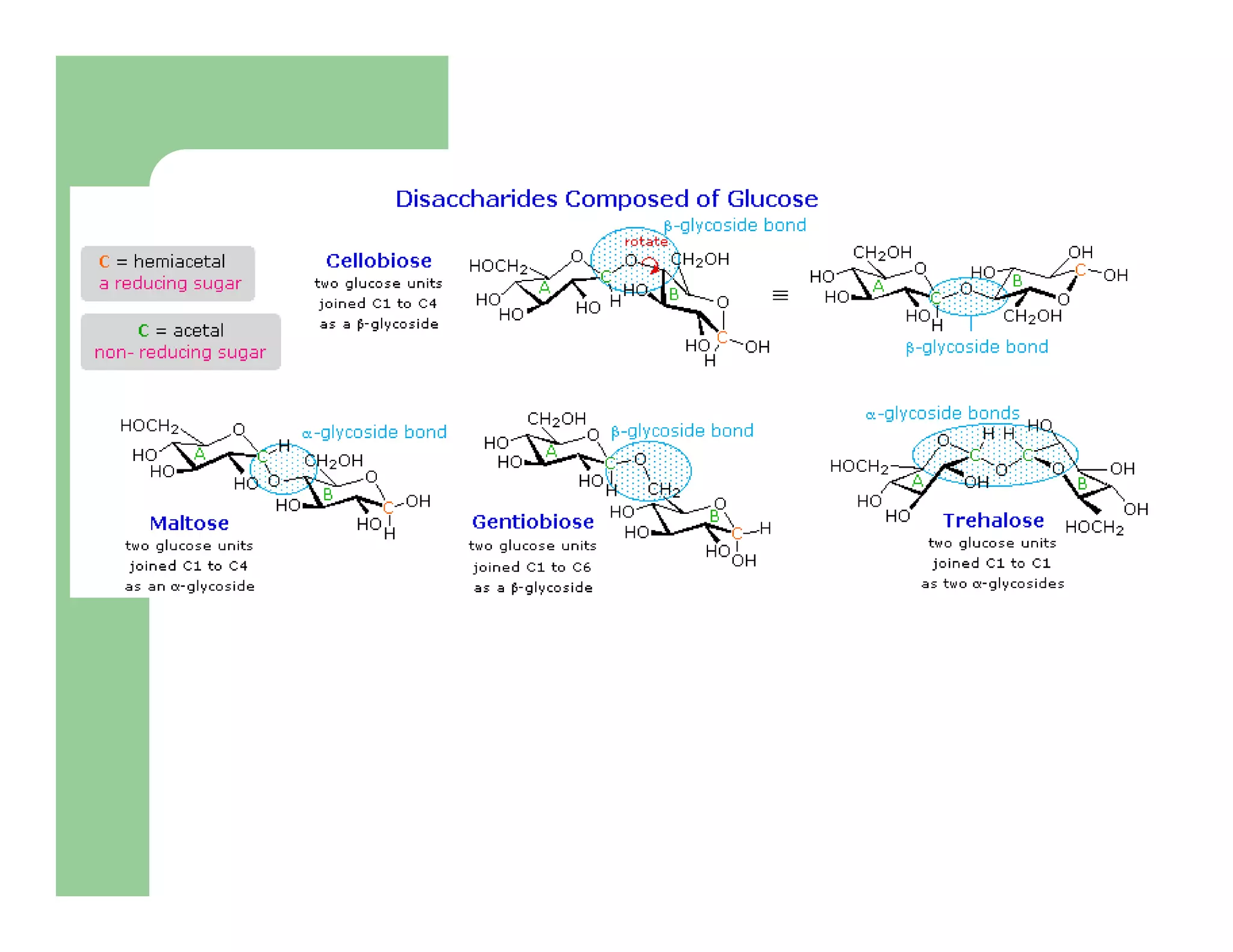
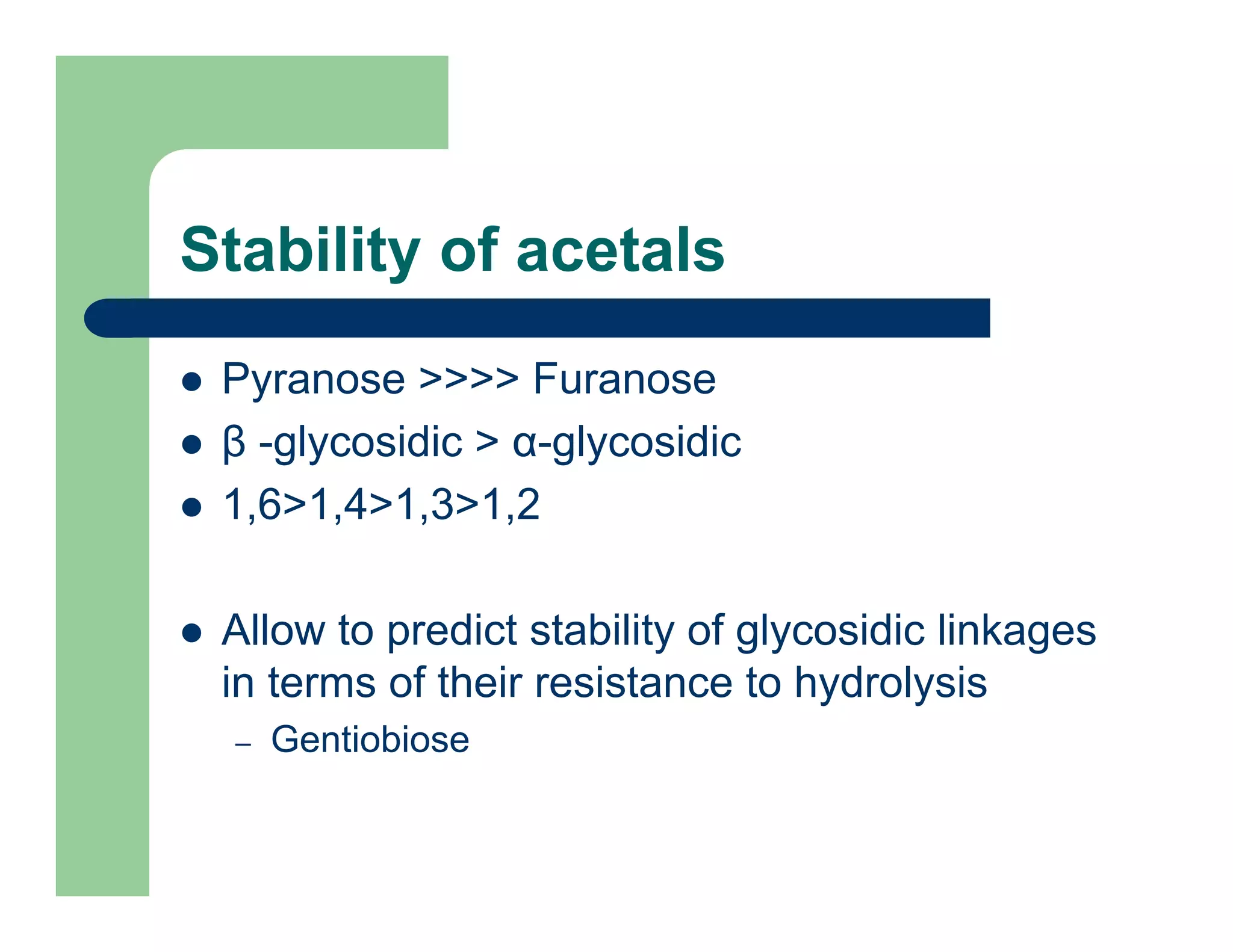
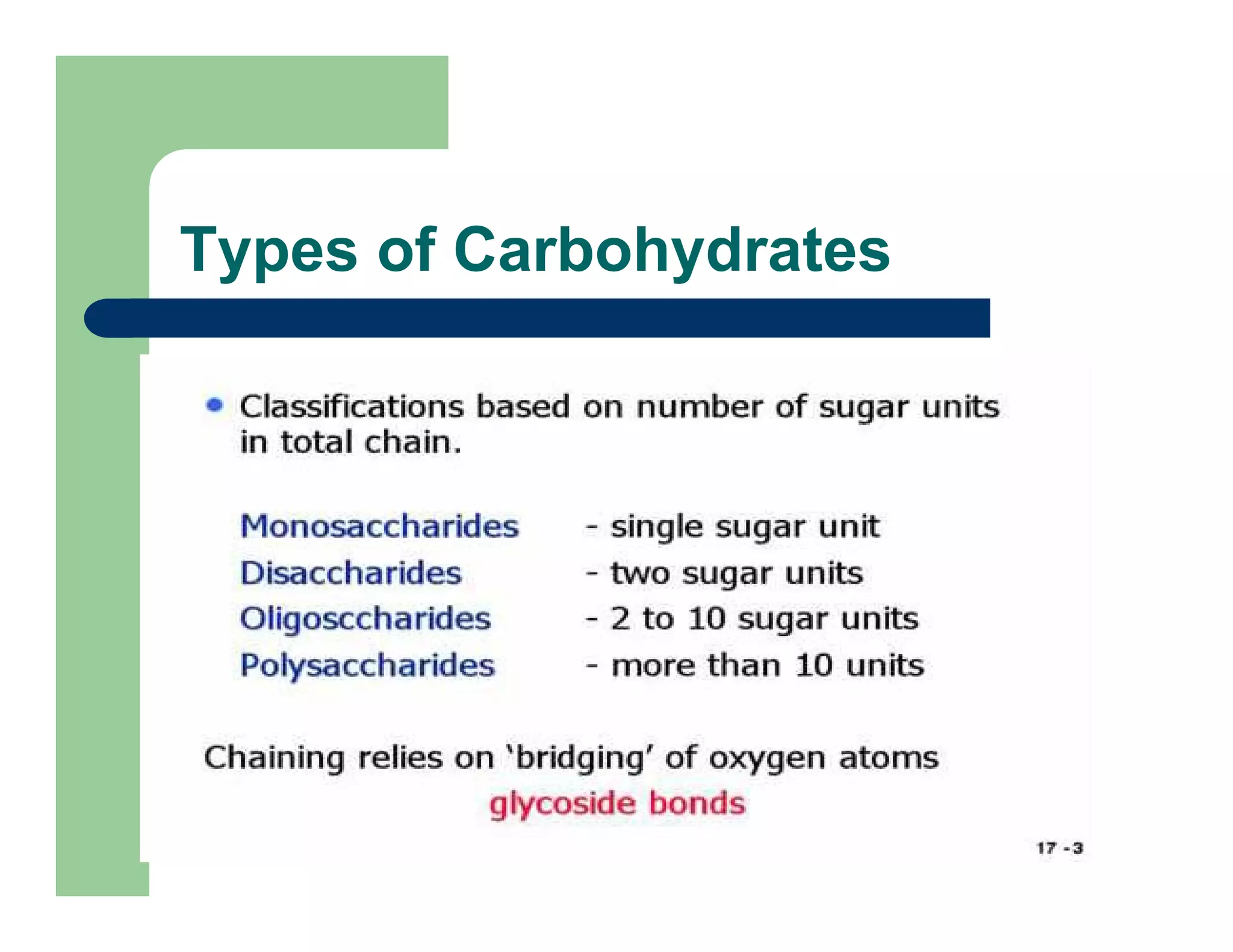
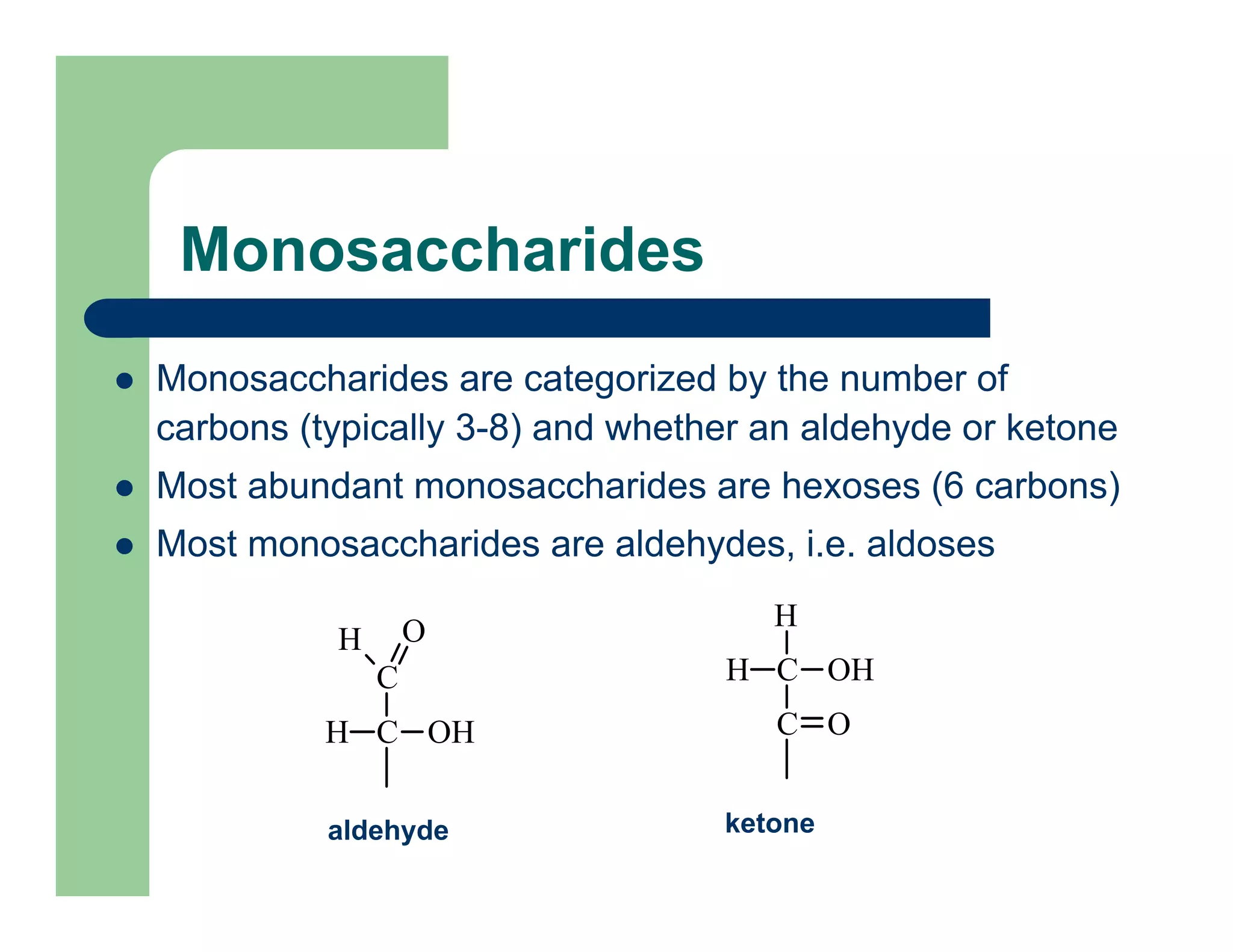
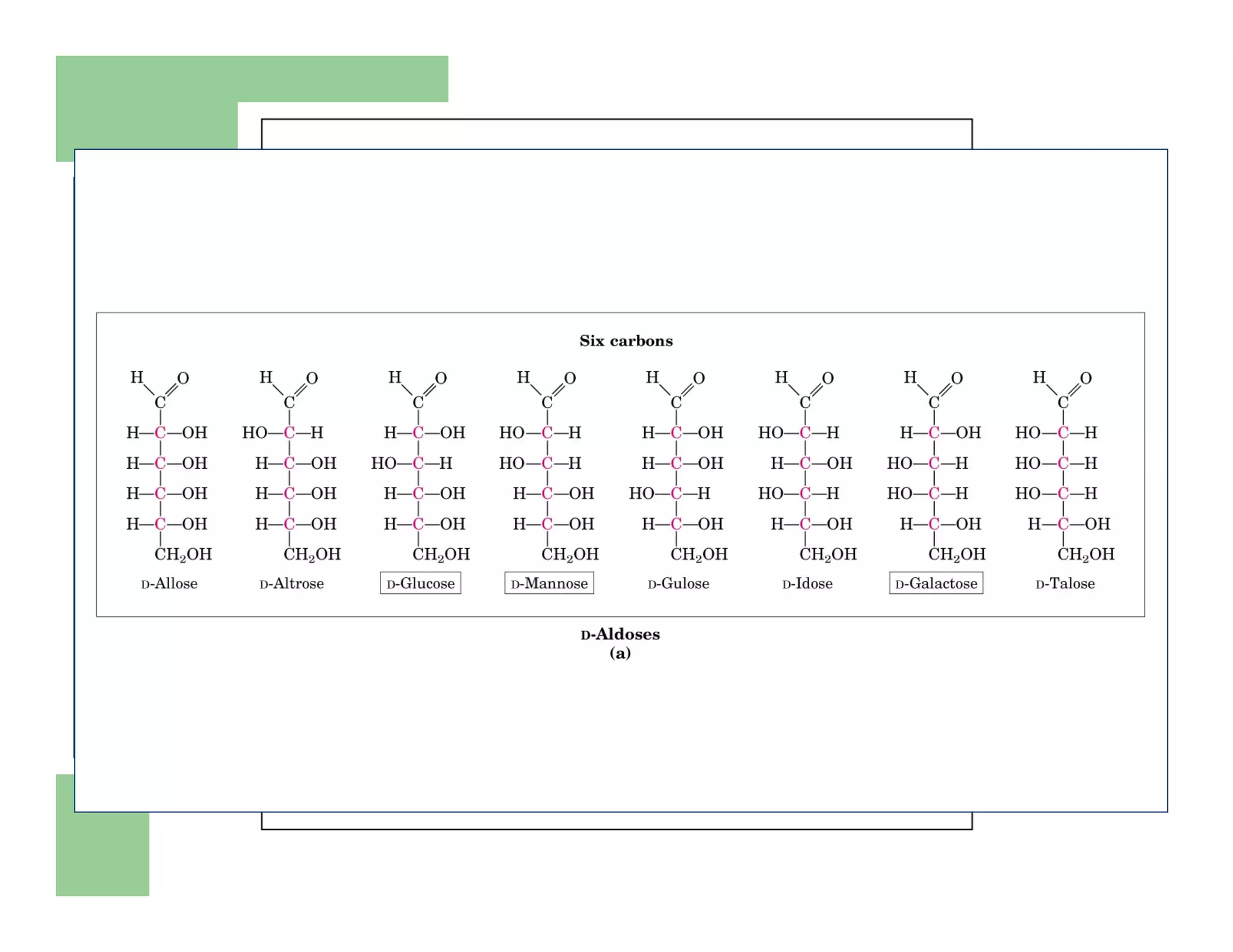
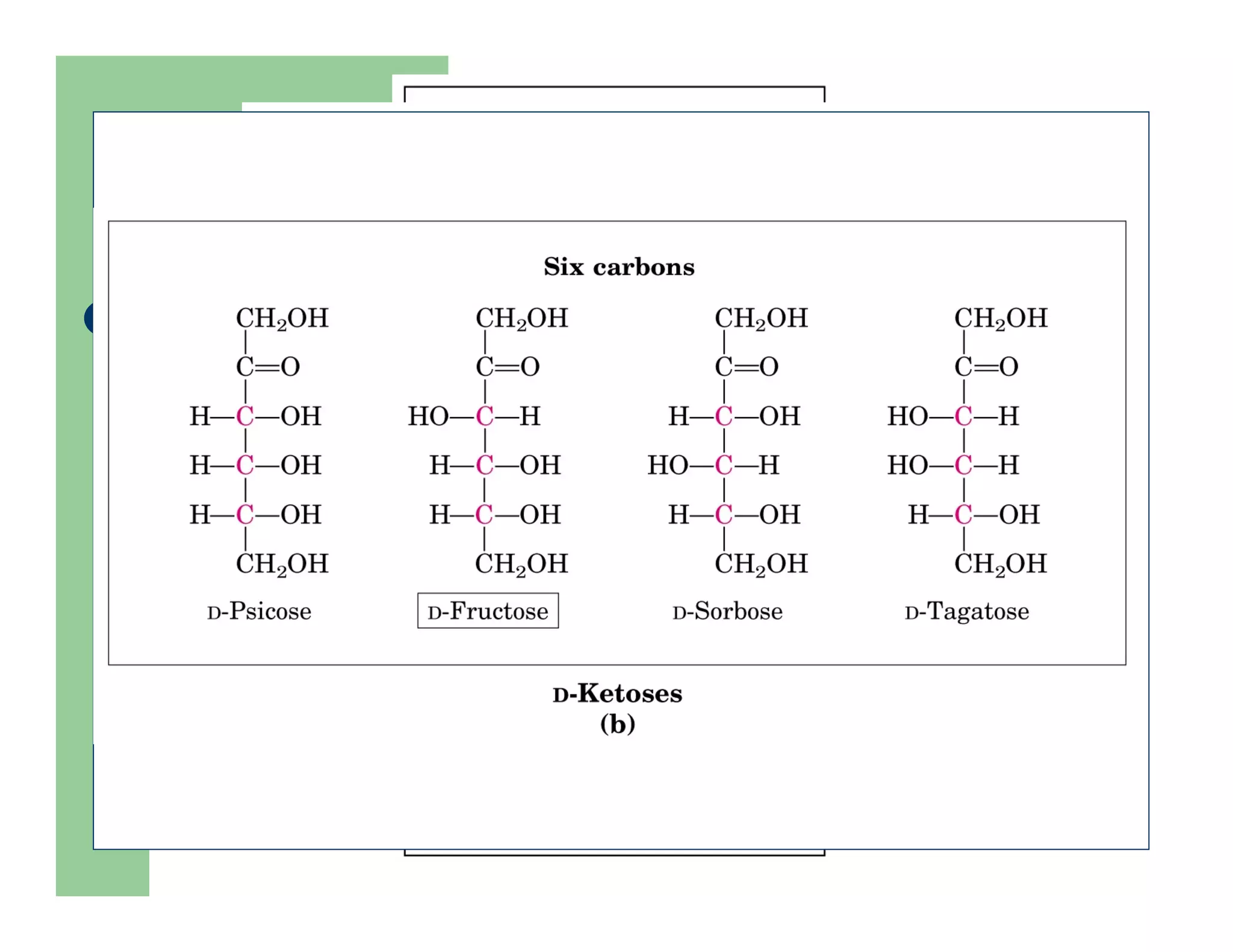
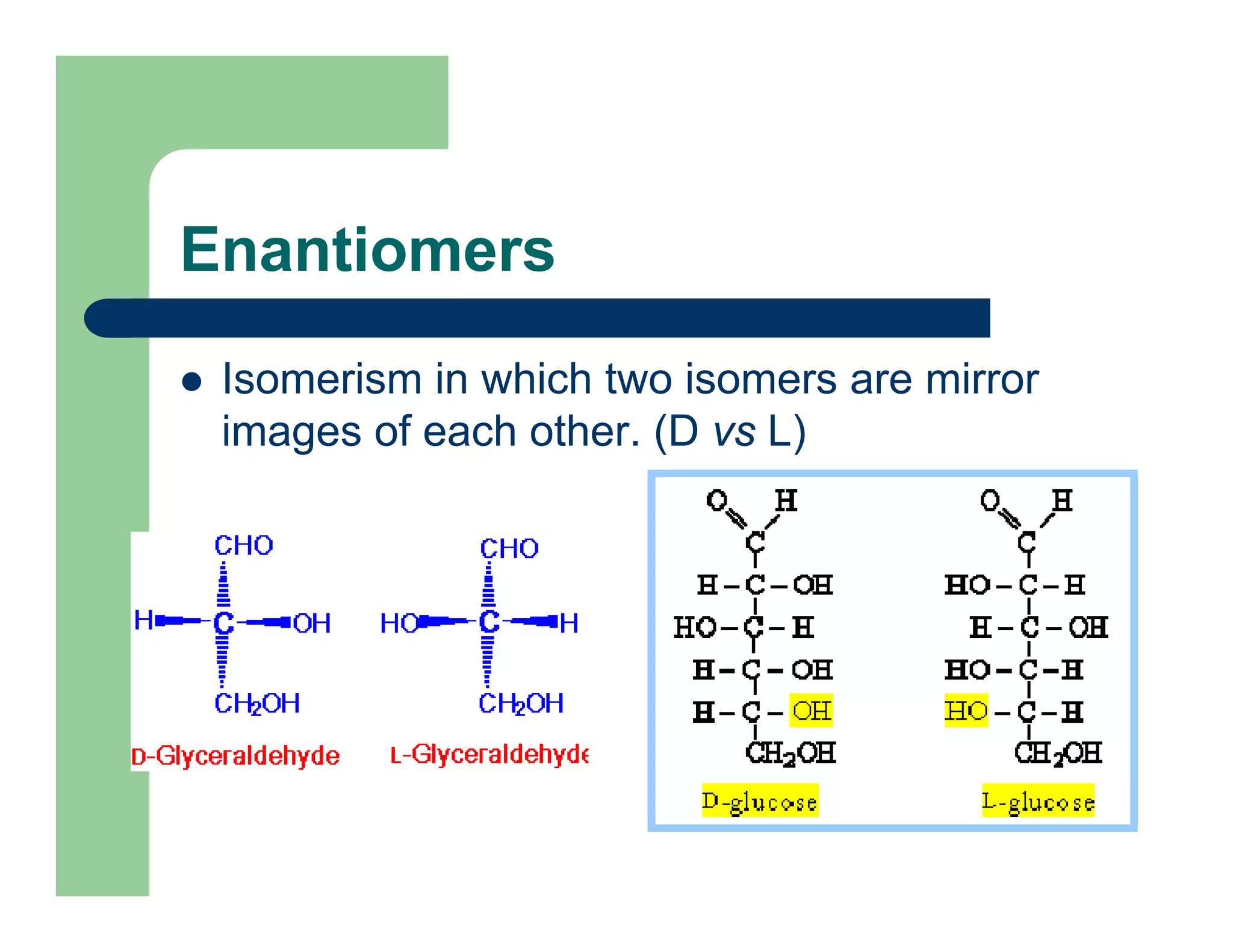
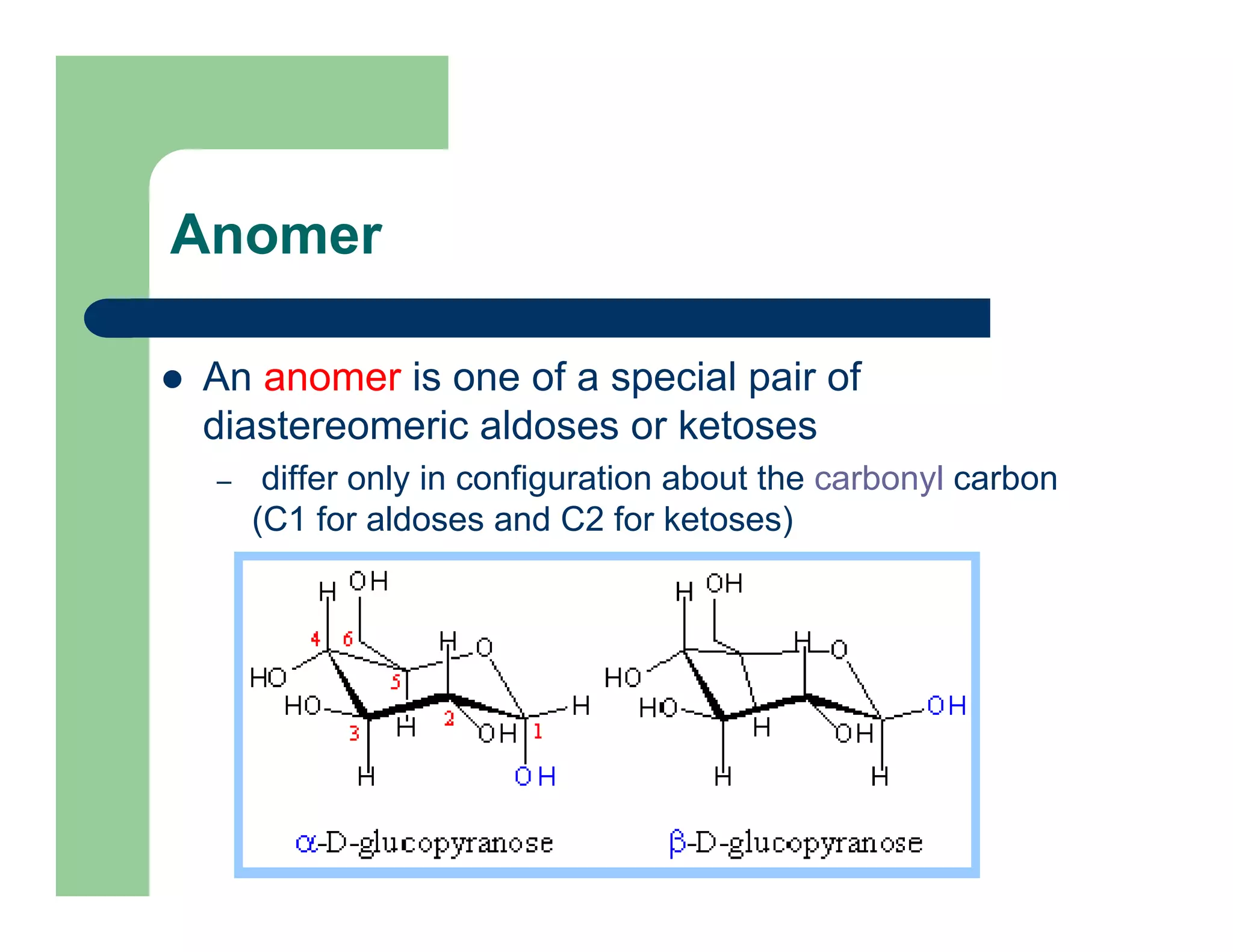
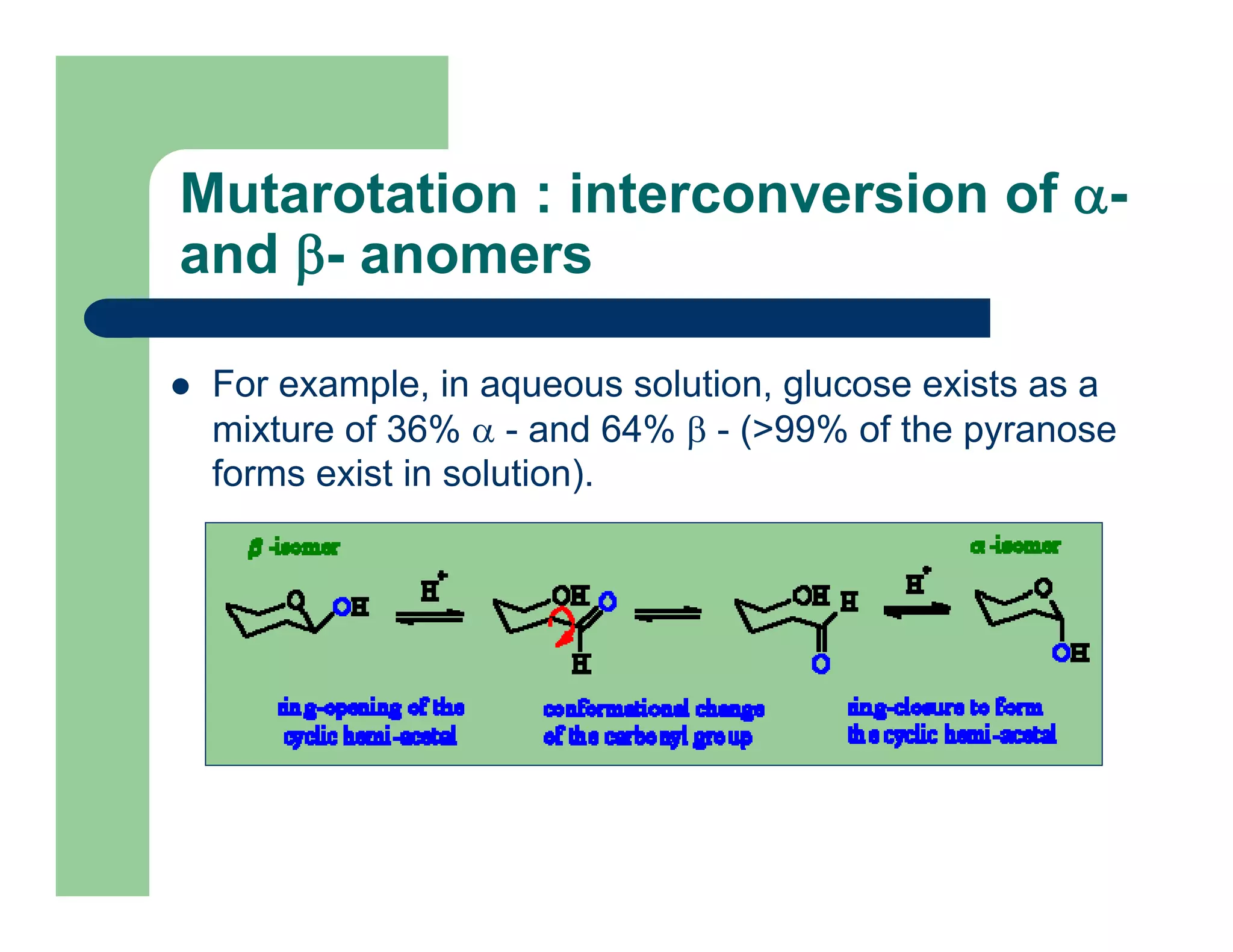
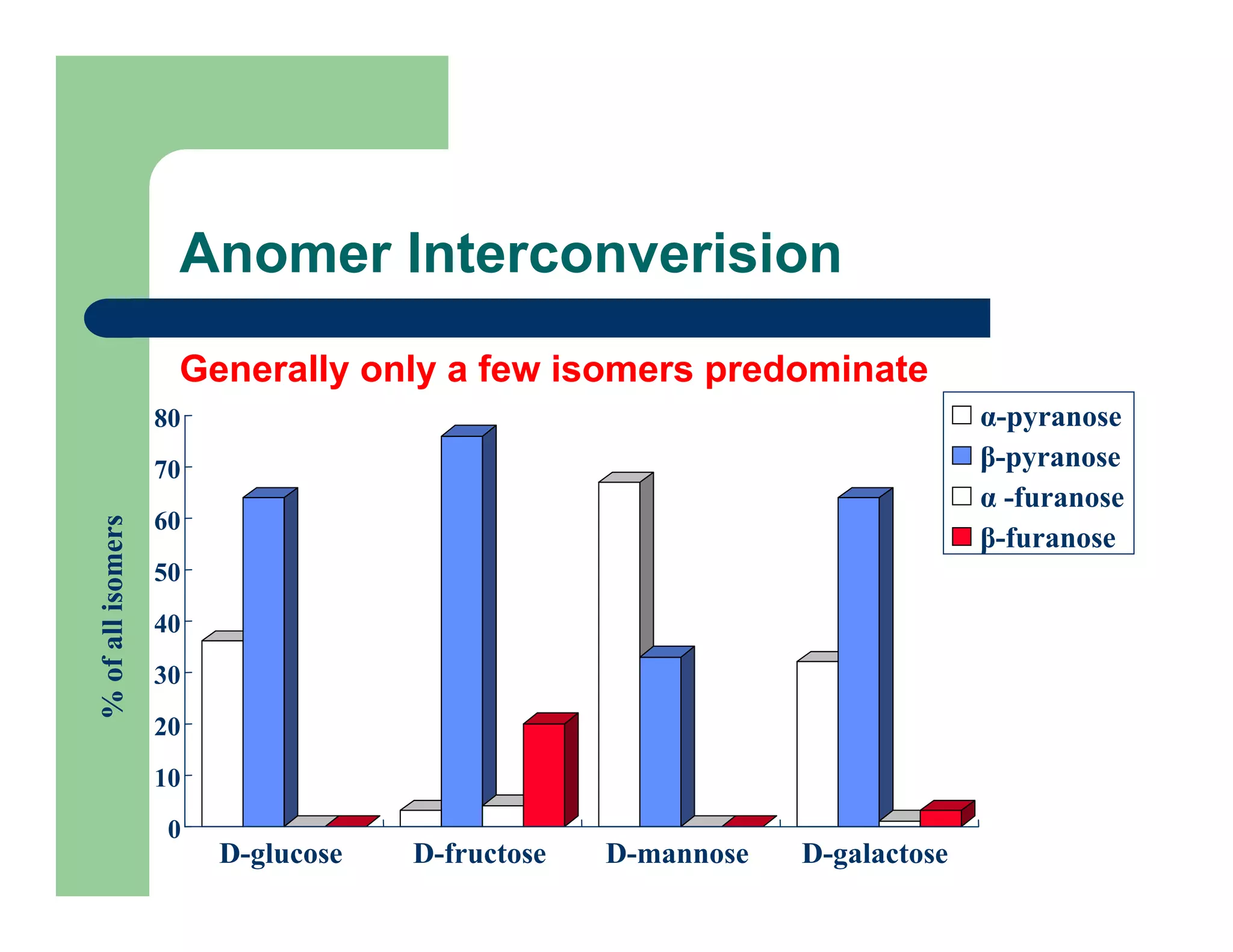
Carbohydrates are polyhydroxy compounds that contain a carbonyl group and are composed of carbon, hydrogen, and oxygen. The three main types are monosaccharides, oligosaccharides, and polysaccharides. Monosaccharides like glucose and fructose exist as both open-chain and ring forms, with the ring forms being more stable. Carbohydrates undergo reactions like isomerization, oxidation, reduction, and acetal formation involving their carbonyl groups. They can exist in several isomeric forms differing in stereoconfiguration and anomeric form.










![TIME
+57.2o
+112o
+19o
pure α-D-(+)-glucopyranose1
pure β-D-(+)-glucopyranose2
[α]D
66% β
34% α
(min)](https://image.slidesharecdn.com/carbohydrates2-150111070109-conversion-gate02/75/Carbohydrates-Biochemistry-21-2048.jpg)