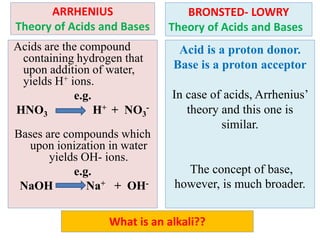
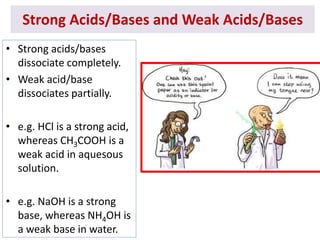
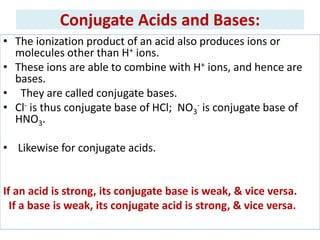
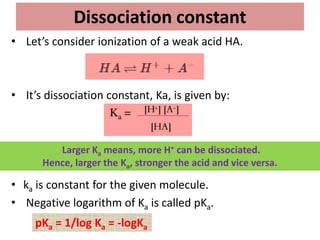
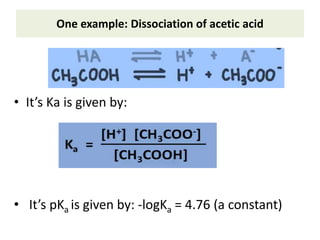
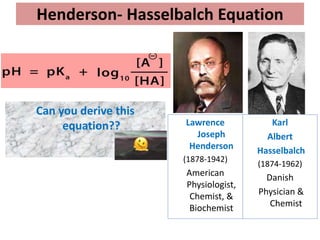
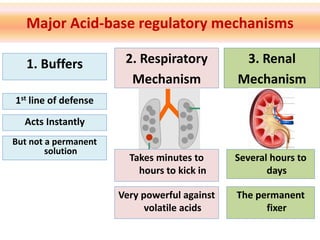
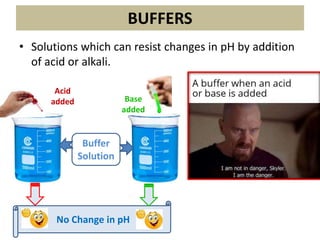
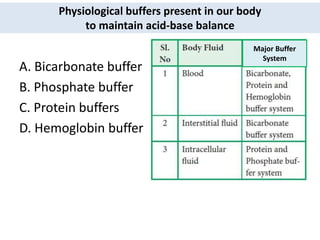
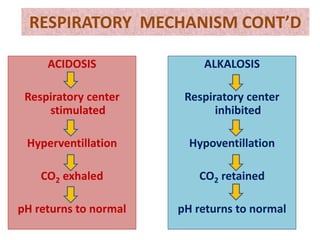
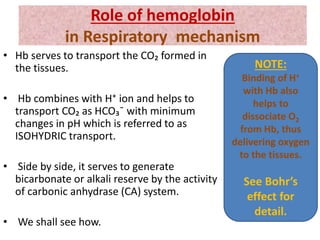
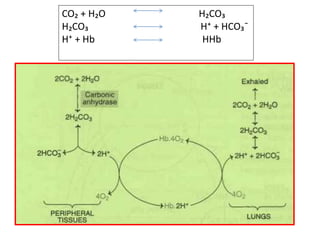
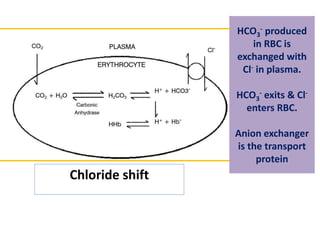
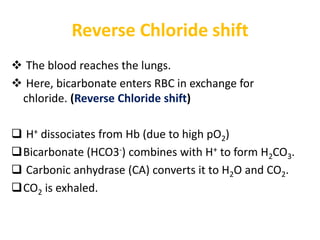
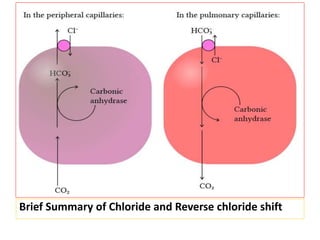
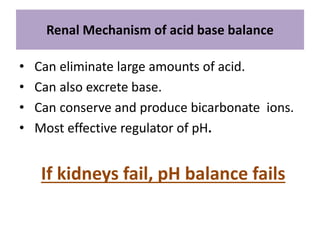
The document discusses acid-base balance and the mechanisms that regulate it in the human body. It covers topics such as the definition of pH, acids and bases according to different theories, strong vs weak acids and bases, buffers and their role in maintaining pH, and the three major mechanisms of acid-base regulation - buffers, respiratory system, and renal system. The respiratory system acts within minutes to change breathing rate and exhale more carbon dioxide to return pH to normal levels in response to acidosis or alkalosis. The renal system takes longer but is the primary mechanism for regulating acid-base balance over hours to days.


![pH
• Potential/Power of hydrogen.
• Sørensen introduced the
concept of pH to measure the
acidity and alkalinity of the
solution.
• Is a negative logarithm of [H+].
pH = 1/ log[H+] = -log[H+]
• Ranges from 0 to 14.
• pH 7 is considered neutral.
Sørensen (1868-1939)
Danish Chemist](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-3-320.jpg)


![Other forms of Henderson-Hasselbalch equation
e.g. If CH3COOH dissociates to
Ch3COO- and H+,
CH3COO- can combine with Na+ to
form a salt CH3COONa.
Then,
pH = pKa + log [salt] / [Acid]
Similarly,
pH = pKa+ log [salt]/[Base]](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-12-320.jpg)
![New (& more commonly used) definition of pKa
When dissociated [A-]= undissociated [HA],
then pH = pKa.
Therefore:
pKa is that pH where half of the acid (or
ions) exist in dissociated form.](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-13-320.jpg)
![Example of acetic acid again
• At pH 4.76, half of the CH3COOH is
dissociated to CH3COO- and H+
i.e. ([CH3COOH]= [CH3COO-]).
• Below 4.76 (i.e. pH < pKa), acetic
acid does not dissociate much,
- i.e. [CH3COOH]> [CH3COO-].
• Above 4.76 (i.e. pH > pKa), more
than half of acetic acid is
dissociated,
- i.e. [CH3COOH]< [CH3COO-].
pKa = 4.76](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-14-320.jpg)

![Production of acids is a physiological process
• Carbonic acids (aerobic
glycolysis), lactic acids
(anaerobic glycolysis), sulfuric
acid, phosphoric acid (sulfo-
and phospho-proteins), keto
acids etc are produced on
regular basis.
Volatile acids i.e.
Carbonic acid is produced
around 20,000 meq/day.
Fixed (non-volatile) acids
like lactic and sulfuric
acids are produced around
80 meq/day].
No wonder our urine is acidic (except for pure
vegan diet where production of alkali
overwhelms acid production)](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-17-320.jpg)

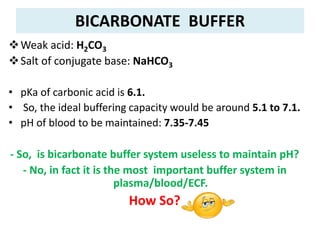
![pKa is not the only factor that determines buffering
capacity...
• To illustrate this, let’s have a look at Henderson-
Hasselbalch equation again:
• In case of bicarbonate buffer:
or
Or, pH = 6.1 + log [NaHCO3]/[H2CO3]
pH = pKa + log [NaHCO3]
[H2CO3]
pH = pKa + log [HCO3
-]
[H2CO3]](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-25-320.jpg)
![• Normal, [NaHCO3] in plasma= 27meq/L
• Normal, [H2CO3] in plasma = 1.35 meq/L
• Therefore,[NaHCO3]/ [H2CO3]= 20.
• Let’s put this value in our earlier equation.
pH = 6.1 + log 20
= 6.1 + 1.3
= 7.4.
Bicarbonate buffer is
therefore
sometimes
referred to as:
Good physiological
buffer
([Salt]/[Acid] = 20),
AND
Weak/Poor chemical
buffer
(pKa = 6.1)
So, although the pKa value of bicarbonate
buffer is not favorable to maintain the pH
between 7.35 & 7.45, its high [Salt]/[Acid]
concentration (= 20), more than
compensates for it to act as a good ECF
buffer.](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-26-320.jpg)
![Practical convention
• It is difficult to measure [H2CO3] in laboratory.
• But pCO2 can be easilymeasured.
• And it has been calculated that:
[H2CO3]= 0.03 X pCO2
• Therefore, we can write the Henderson-Hasselbalch
equation for bicarbonate buffer as:
We shall use this latter equation frequently.
pH = pKa + [HCO3]/0.03 X pCO2](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-27-320.jpg)
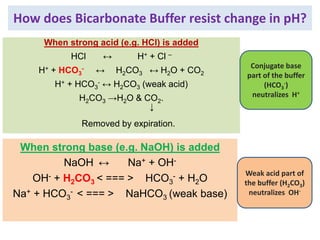

![PHOSPHATE BUFFER
Weak Acid: NaH2Po4
Salt of Conjugate base: Na2HPO4
• Buffer action mainly important intra-cellular.
• Also plays important role in buffering renal tubular fluid.
pKa = 6.8
[Salt]/[Acid] = 4:1](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-30-320.jpg)
![Buffering Efficiency in ECF
pKa = 6.8; [Salt]/[Acid] = 4:1
Therefore,
pH = pKa + log [Salt]/[Acid]
= 6.8 + log 4
= 6.8 + 0.6
= 7.4
So, it is still an efficient buffer
despite low [salt]:[Acid]
(compared to bicarbonate
buffer)
Phosphate Buffer is
sometimes called as:
Good Chemical Buffer
(pKa = 6.8),
AND
Poor Physiological Buffer
([Salt]:[Acid] = 4)](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-31-320.jpg)
![Point to note:
In fact, Phosphate buffer is efficient across even wider pH range
• Phosphate buffer has 3 PKa values, as it has 3
dissociable H+ ions.
One of these pKa
value (6.8) and
[Salt]:[Acid] is
suitable for it to
act as biological
buffer
H₃PO₄ H⁺ + H₂PO₄¯ pKₐ = 1.96
H₂PO₄¯ H⁺ + HPO₄¯ ¯ pKₐ = 6.8
HPO₄¯ ¯ H⁺ + PO ₄° pKₐ = 12.4](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-32-320.jpg)
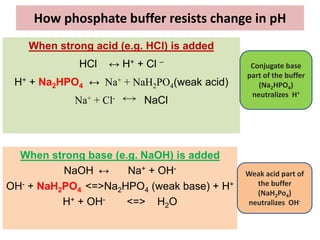

![Protein Buffers
Proteins are among the most plentiful buffers in the
body because of their high concentrations, especially
within the cells.
Approximately 60-70% of the total chemical buffering
of the body fluids is inside the cells, and most of this
results from the intracellular proteins.
[In addition to the high concentration of proteins in the
cells, another factor that contributes to their buffering
power is the fact that the pKa of many of these protein
systems are fairly close to 7.4]](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-35-320.jpg)
![Proteins act as buffers because of their
amphoteric nature.
Buffering capacity of protein depends on the
pKₐ value of ionizable side chains.
The amino acid residues having pKₐ close to
7.4 are the most effective in buffering.
The most effective group is histidine
imidazole group.
Proteins especially albumin, account for
about 95% of the non-bicarbonate buffer
value of plasma.
[Each albumin contains 16 histidine residues
which make it an effective buffer]
Assignment:
Name some
important
intracellular
protein
buffers](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-36-320.jpg)




![Details during Renal system
4 major ways by which the renal regulation operates :
A. Excretion of H⁺ (& production of bicarbonates)
B. Reabsorption of bicarbonate
C. Acidification of monohydrogen phosphate [May also
be called: Excretion of titrable acid]
D. Secretion of ammonia (NH₄⁺ ions)
8](https://image.slidesharecdn.com/acidbasebalanceuglatest-230407075407-64771a7a/85/ACID-BASE-BALANCE-UG-latest-pptx-49-320.jpg)
