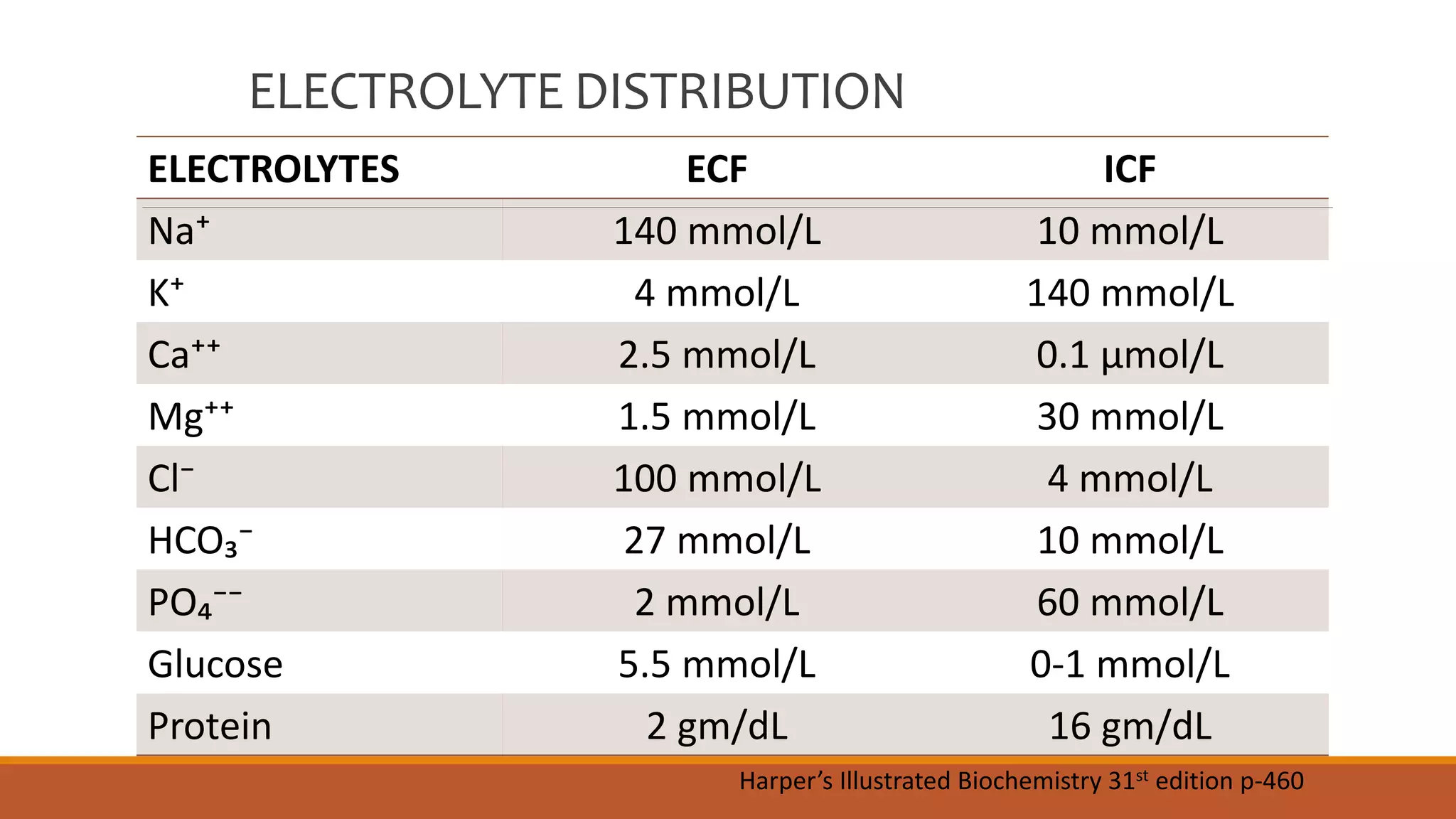
Major electrolytes such as sodium, potassium, calcium, magnesium, chloride, bicarbonate, and phosphate are distributed differently between the extracellular fluid (ECF) and intracellular fluid (ICF) compartments. Calcium homeostasis is tightly regulated by parathyroid hormone (PTH), vitamin D, and calcitonin which act to increase or decrease calcium resorption in bones and reabsorption in kidneys. Hypocalcemia and hypercalcemia can result from disorders of the parathyroid glands or kidneys. Similarly, phosphate levels are regulated by PTH, vitamin D, and calcitonin to affect bone levels, and disorders can cause hypo- or hyperphosphatemia. Magnesium is also regulated and




![Assessment of Calcium Status
Direct method: Measure free Ca⁺⁺
conc.
Indirect method: Measure total
calcium conc. & albumin conc.
T [Ca] ↑↑
[Alb] ↑↑
Free Ca normal
No symptom
T [Ca] normal
[Alb] ↓
Free Ca ↑
Hypercalcaemia
T [Ca] normal
[Alb] ↑
Free Ca ↓
Hypocalcaemia
Adjusted Calcium level: In
abnormal serum albumin
concentration
Total [Ca] + 0.02X(47-A) gm/L
[Total body albumin= 47 gm
1 gm albumin binds with 0.02
mmol/L of Ca]](https://image.slidesharecdn.com/electrolyteshomeostasis2-210120144912/75/Major-Electrolytes-Their-Homeostasis-Part-2-6-2048.jpg)

![Regulation of Plasma Ca Homeostasis
Parathyroid Hormone (PTH): ↑Ca⁺⁺
Vitamin D/ Calcitriol [1,25(OH)₂D₃]: ↑Ca⁺⁺
Calcitonin: ↓Ca⁺⁺
Magnesium: ↓Mg⁺⁺ prevents PTH release & cause ↓Ca⁺⁺
Plasma binding: Approximately 40% of plasma Ca is
bound to plasma protein & remaining free Ca is
biologically active. Labs usually measure total plasma Ca.
So total Ca for albumin needs to be corrected.](https://image.slidesharecdn.com/electrolyteshomeostasis2-210120144912/75/Major-Electrolytes-Their-Homeostasis-Part-2-8-2048.jpg)









![Regulation of Phosphate
The amount of phosphate in the blood affects the
level of calcium in the blood
Calcium and phosphate in the body react in opposite
ways: as blood calcium levels rise, phosphate levels fall
Parathyroid Hormone (PTH): ↓PO₄³⁻
Vitamin D/ Calcitriol [1,25(OH)₂D₃]: ↑PO₄³⁻
Calcitonin: ↓PO₄³⁻](https://image.slidesharecdn.com/electrolyteshomeostasis2-210120144912/75/Major-Electrolytes-Their-Homeostasis-Part-2-18-2048.jpg)




