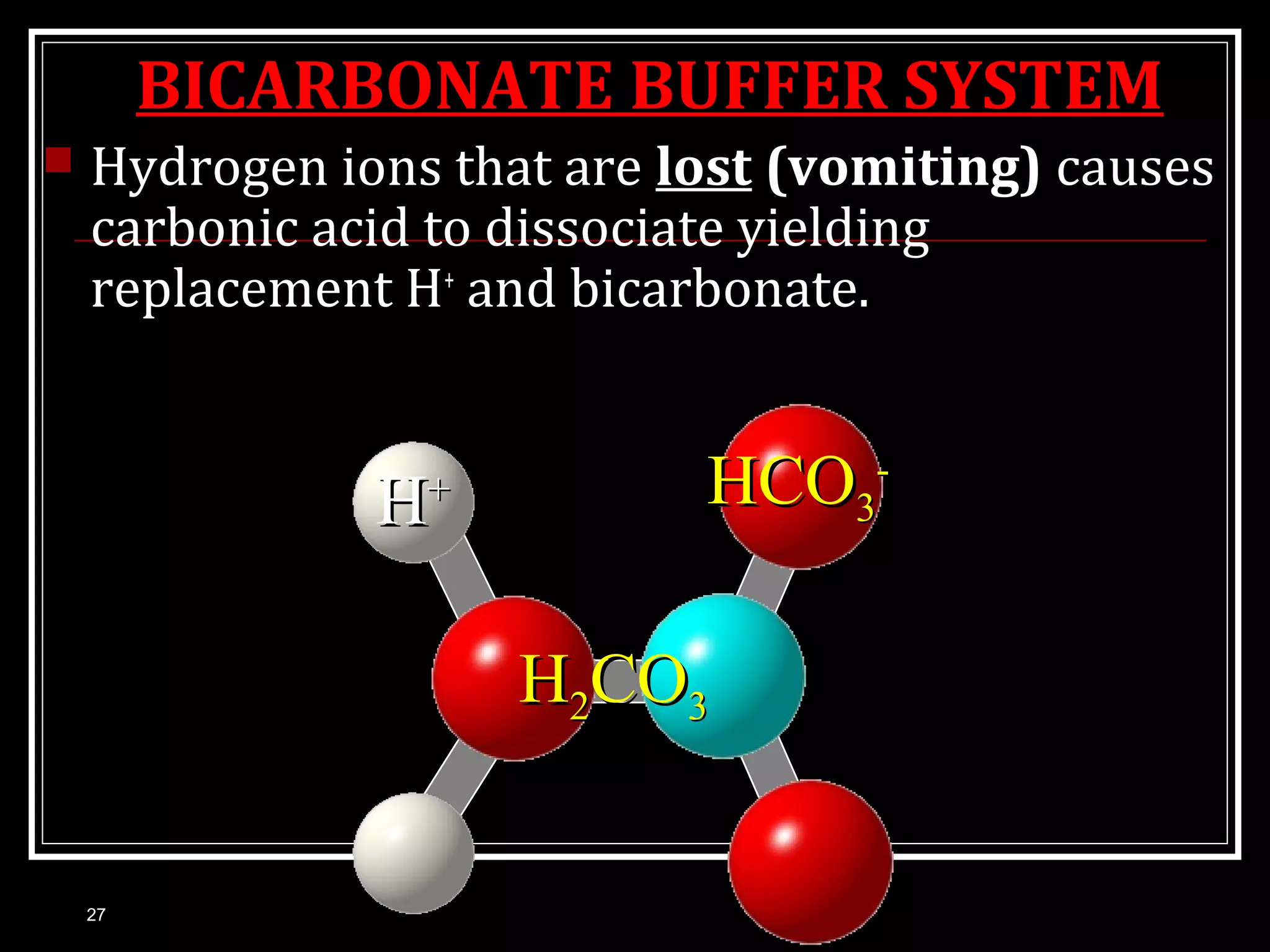
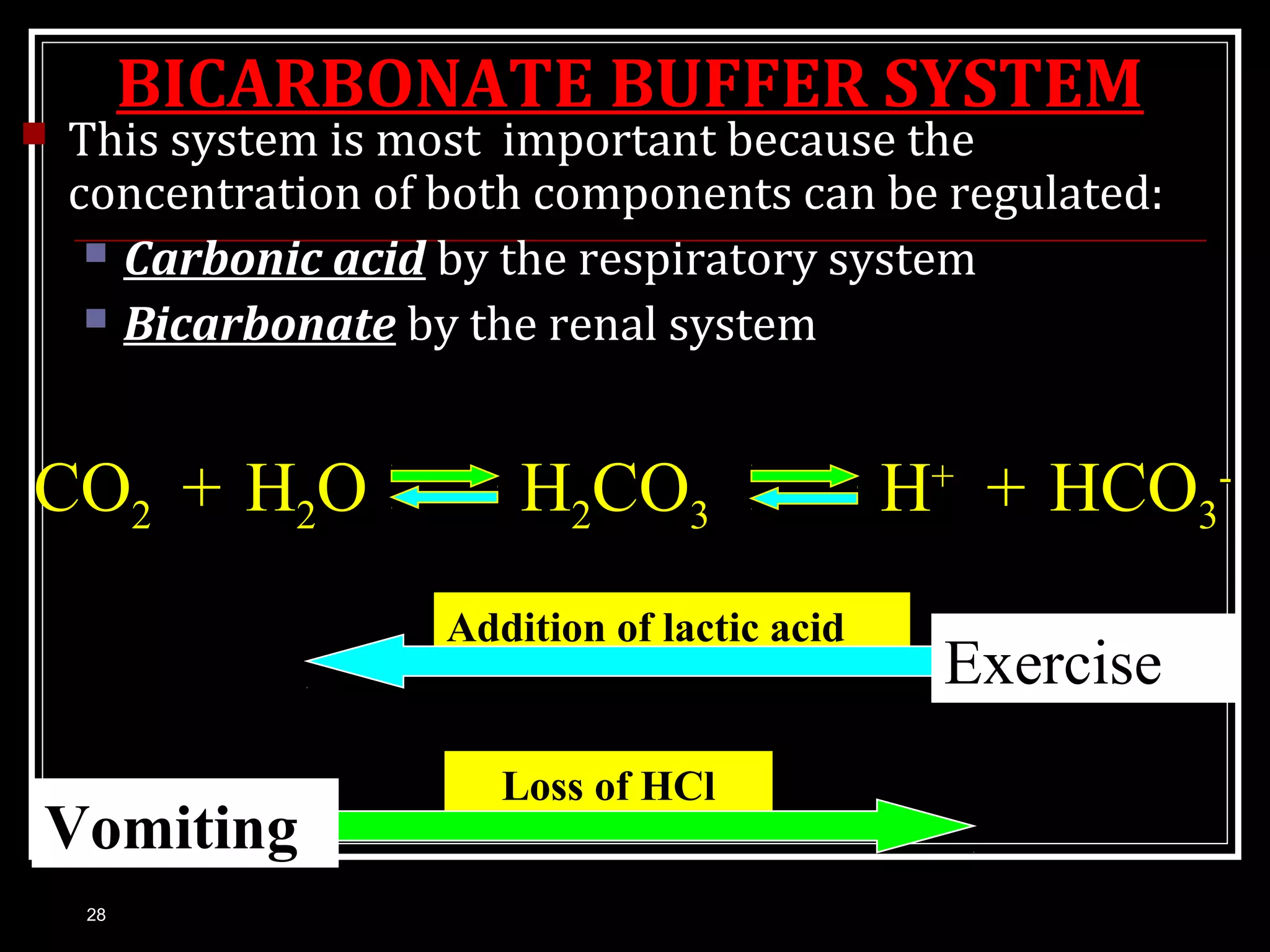
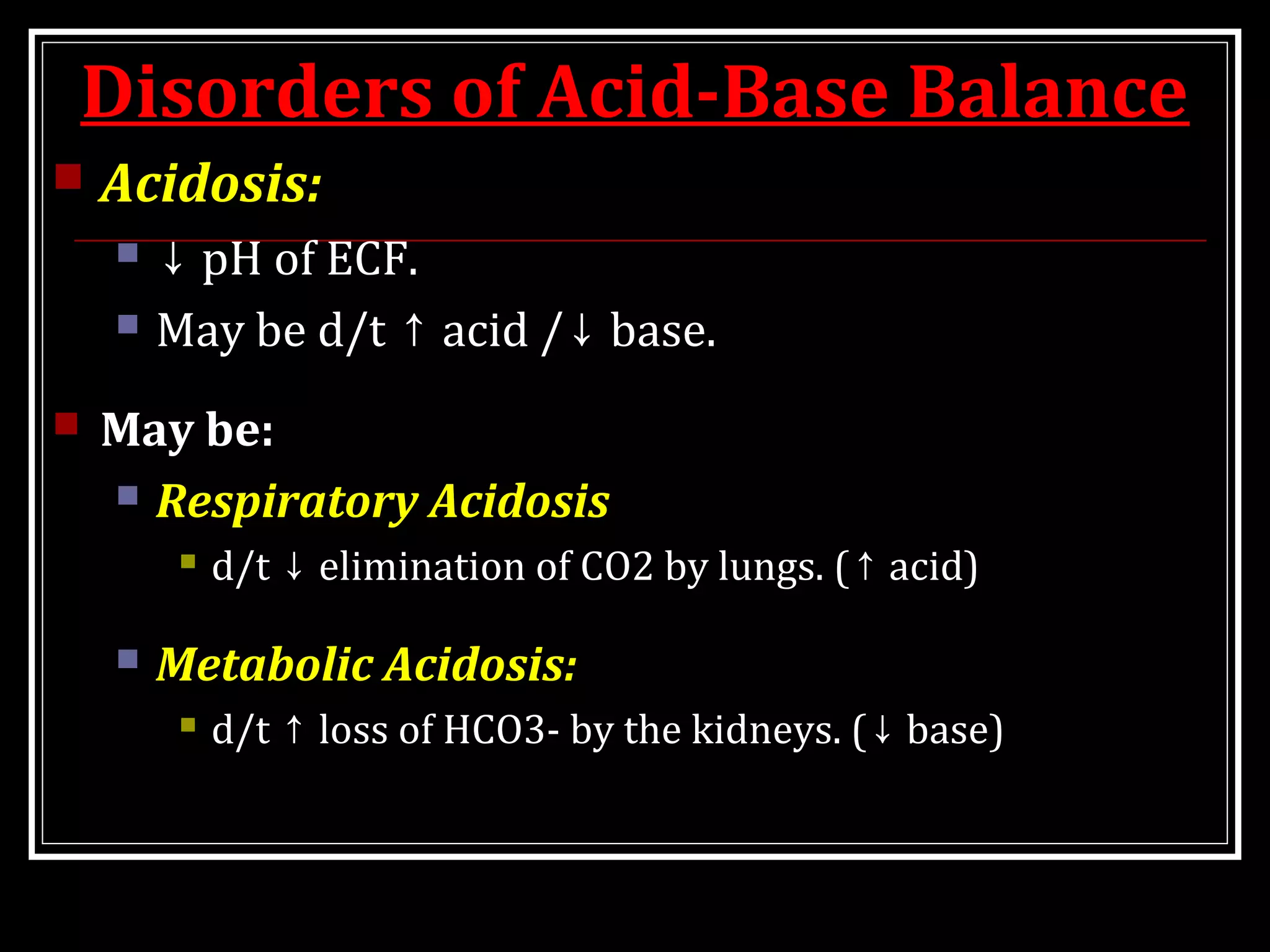
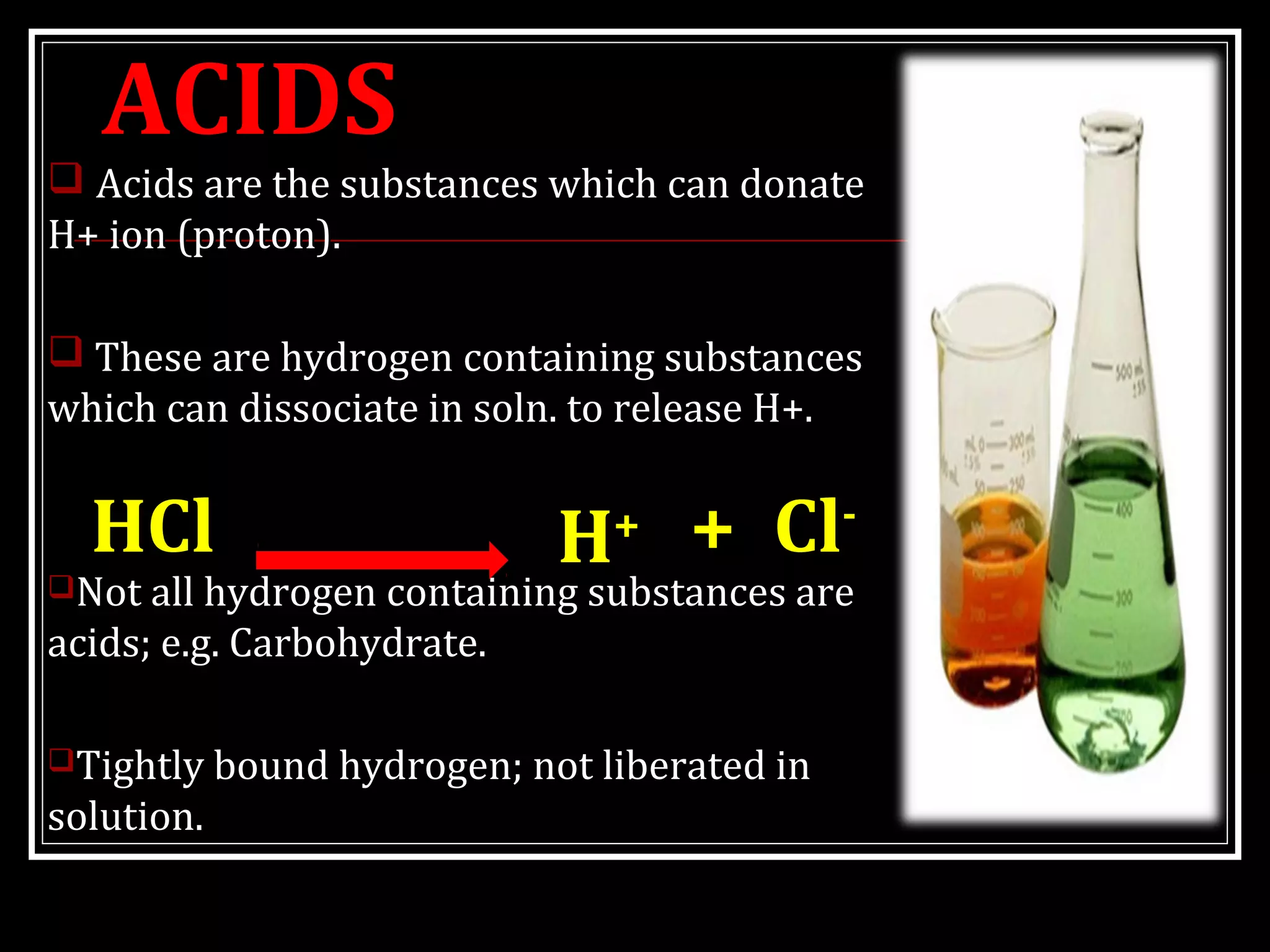
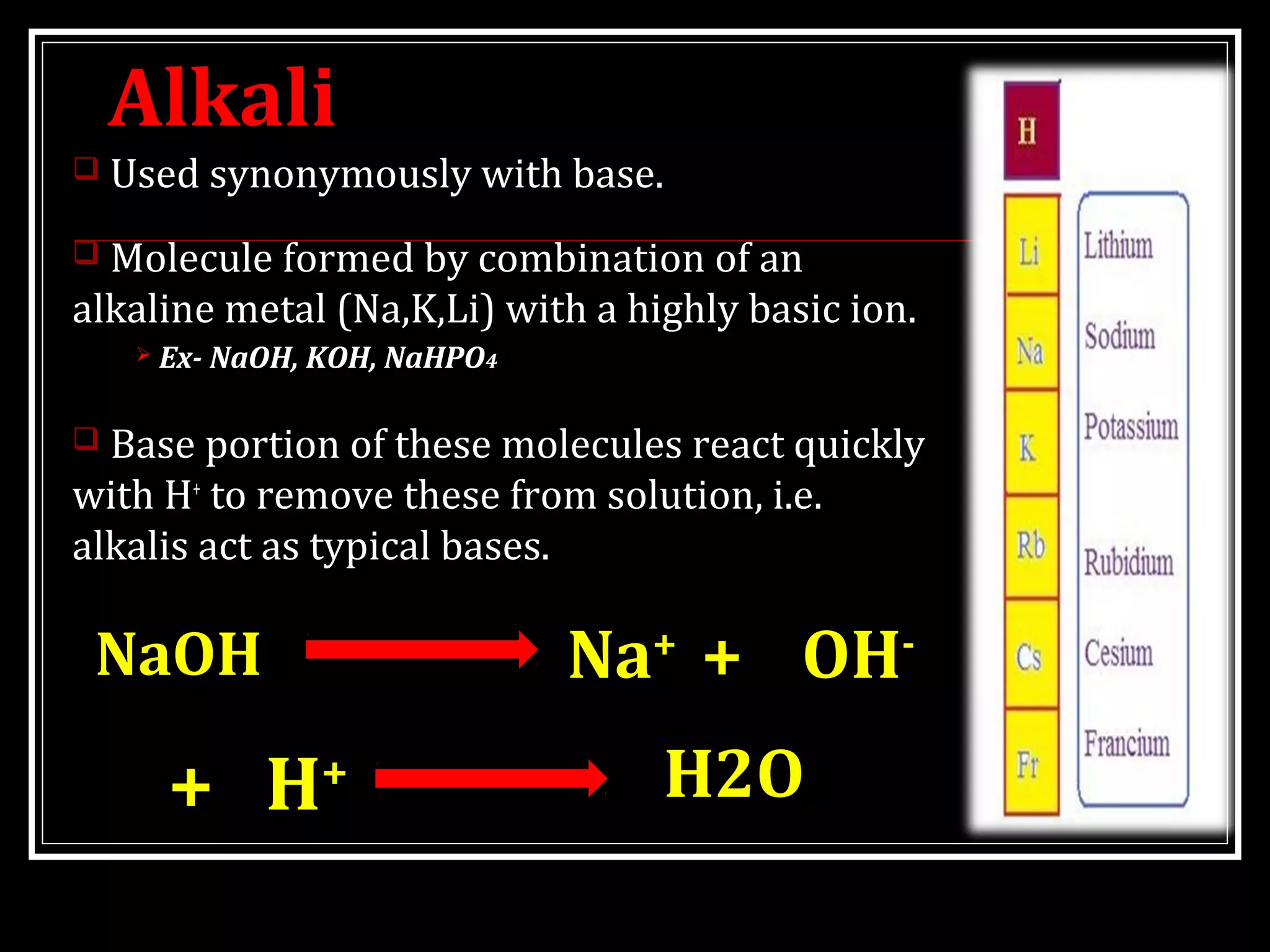
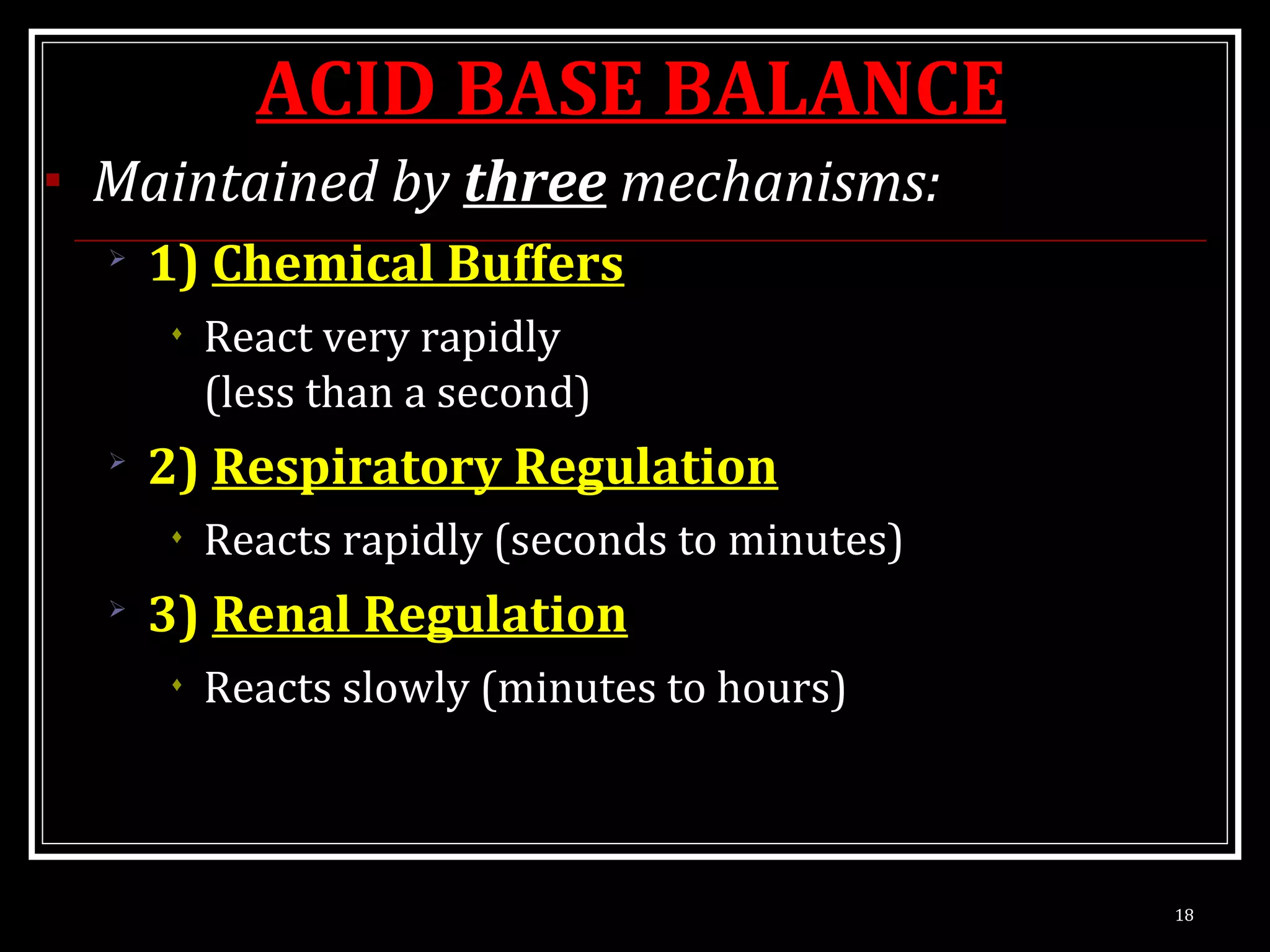
Dr. Nilesh Kate discusses acid-base balance. He defines acids as substances that can donate H+ ions and bases as substances that can accept H+ ions. Important physiological acids include carbonic, phosphoric, pyruvic, and lactic acids. Important bases include bicarbonate and biphosphate. pH is a measure of H+ ion concentration and is maintained between 6.8-8.0 for life. The body maintains acid-base balance through buffer systems, respiratory regulation, and renal regulation.







![PH SCALEPH SCALE
pH = - log [HpH = - log [H++
]]
pH = log 1/ [HpH = log 1/ [H++
]]
pH is inversely related to HpH is inversely related to H++
concentration.concentration.
low pH – indicates high Hlow pH – indicates high H++
concentration.concentration.
high pH – indicates low Hhigh pH – indicates low H++
concentration.concentration.
pH = 4 has 10 times more free HpH = 4 has 10 times more free H++
concentrationconcentration
than pH = 5 and 100 times more free Hthan pH = 5 and 100 times more free H++
concentration than pH = 6concentration than pH = 6
pH range from 1-14.pH range from 1-14.
pH < 7 – AcidicpH < 7 – Acidic
pH > 7 – BasicpH > 7 – Basic
pH = 7 - NeutralpH = 7 - Neutral](https://image.slidesharecdn.com/acidbasebalance-150418134400-conversion-gate02/75/ACID-BASE-BALANCE-10-2048.jpg)





![ACID BASE BALANCEACID BASE BALANCE
Maintenance of the pH of body fluids at a level that
allow optimal function.
pH maintenance means maintaining [H[H++
].].
This involves two important ions which are regulated byThis involves two important ions which are regulated by
various chemical & physiological process:various chemical & physiological process:
H+
HCO3
-](https://image.slidesharecdn.com/acidbasebalance-150418134400-conversion-gate02/75/ACID-BASE-BALANCE-16-2048.jpg)





![Most Effective Buffer.
Henderson-Hasselbalch equation.
HA H+
+ A-
By the law of mass action, at equilibrium
K = [H+][A-]/ [HA]
----------- K= Dissociation constant of acid.
Saturday, April 18, 2015](https://image.slidesharecdn.com/acidbasebalance-150418134400-conversion-gate02/75/ACID-BASE-BALANCE-23-2048.jpg)
![Henderson- Hasselbalch
equation.
[H+] = K [HA]/[A-]
pH = log 1/ [H+]
Log 1/[H+] = log 1/K +log [A-]/[HA]
pH = pK + log [A-]/[HA]
Thus pH= pK
Thus most effective buffers in the body are those with pK
close to the pH in which they operate.
from this equation it is seen that buffering capacity of
buffer system is greatest when amount of anion[A-] and
undissociated acid [HA] is same.
Saturday, April 18, 2015](https://image.slidesharecdn.com/acidbasebalance-150418134400-conversion-gate02/75/ACID-BASE-BALANCE-24-2048.jpg)