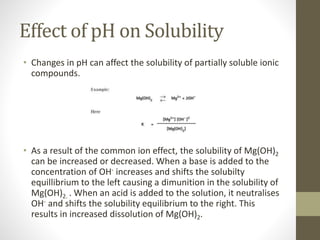
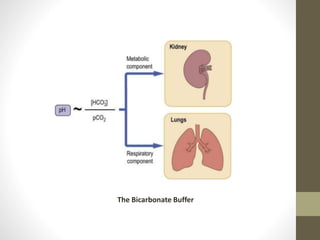
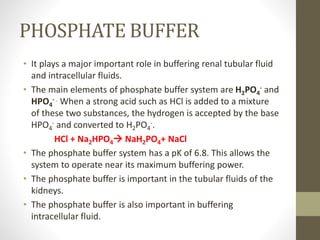
This document provides an overview of buffers in biological systems. It discusses how biological buffers help maintain pH within a narrow physiological range through reversible acid-base reactions. The key types of biological buffers mentioned are bicarbonate, phosphate, proteins, and hemoglobin. Bicarbonate buffers blood pH through reactions involving carbonic acid and carbon dioxide transport. Phosphate buffers intracellular and renal fluid. Proteins and hemoglobin buffer through amino acid side chains that react with hydrogen ions. Maintaining pH is critical for biochemical processes and homeostasis in living organisms.




![Handerson-Hasselbalch Equation
• The pH of a solution containing a weak acid is related to its
acid dissociation constant. The relationship can be stated in
the convenient form of the “ Handerson- Hasselbalch
equation”
pH = Pka+ log10 [A-]
[HA]
• The Handerson- Hasselbalch equation is an expression of
great predictive value in protonic equilibria.](https://image.slidesharecdn.com/buffersinbiologicalsystems-210813115522/85/Buffers-in-biological-systems-5-320.jpg)


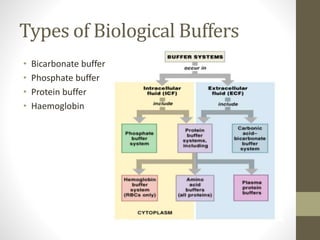



![Buffering in Cells and Tissues
• Amino acids present in proteins in cells and tissues contain
functional groups that act as weak acid and bases.
• The phosphate and bicarbonate buffer systems are most
predominant in biological systems.
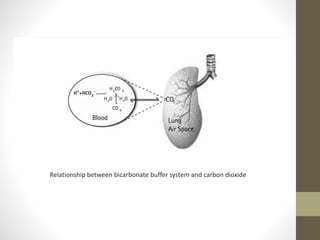
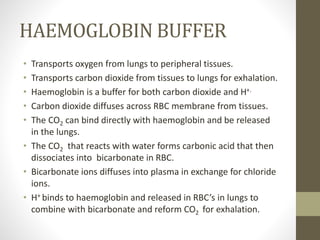
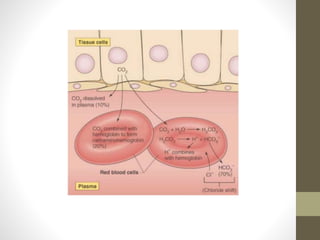
• The bicarbonate buffer system plays an important role in
buffering the blood system where in carbonic acid acts as a
weak acid (proton donor) and bicarbonate act as a conjugate
base (proton acceptor). Their relationship can be expressed as
follows:-
K1 = [H+] [HCO3
-]
[H2CO3]](https://image.slidesharecdn.com/buffersinbiologicalsystems-210813115522/85/Buffers-in-biological-systems-17-320.jpg)