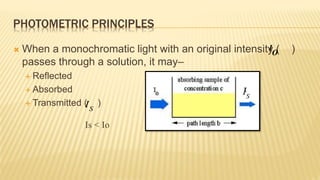
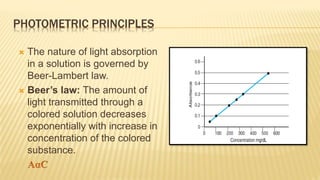
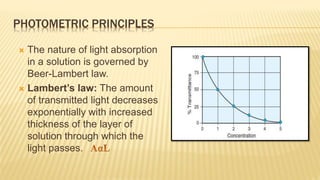
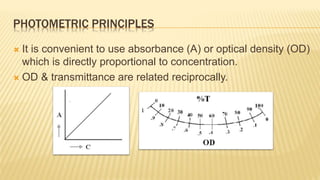
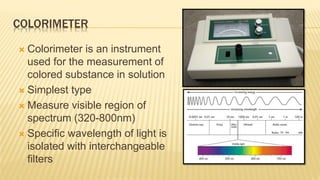
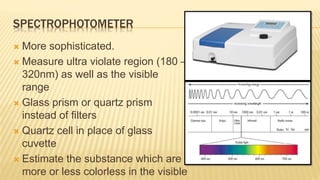
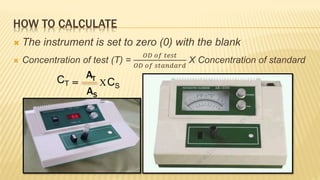
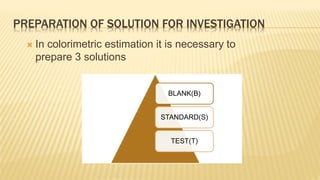
Photometry broadly deals with the study of light absorption by molecules in solution. It is one of the most common analytical techniques used in clinical biochemistry laboratories to measure the intensity of a light beam. Most clinical chemistry reactions involve linking a chemical or enzymatic reaction to the development of a colored product, the intensity of which is then measured photometrically. The amount of light transmitted through a colored solution decreases exponentially with increases in the concentration and thickness of the colored substance, as governed by Beer's and Lambert's laws. Common photometers include colorimeters, which measure visible light, and spectrophotometers, which can measure ultraviolet and visible light.