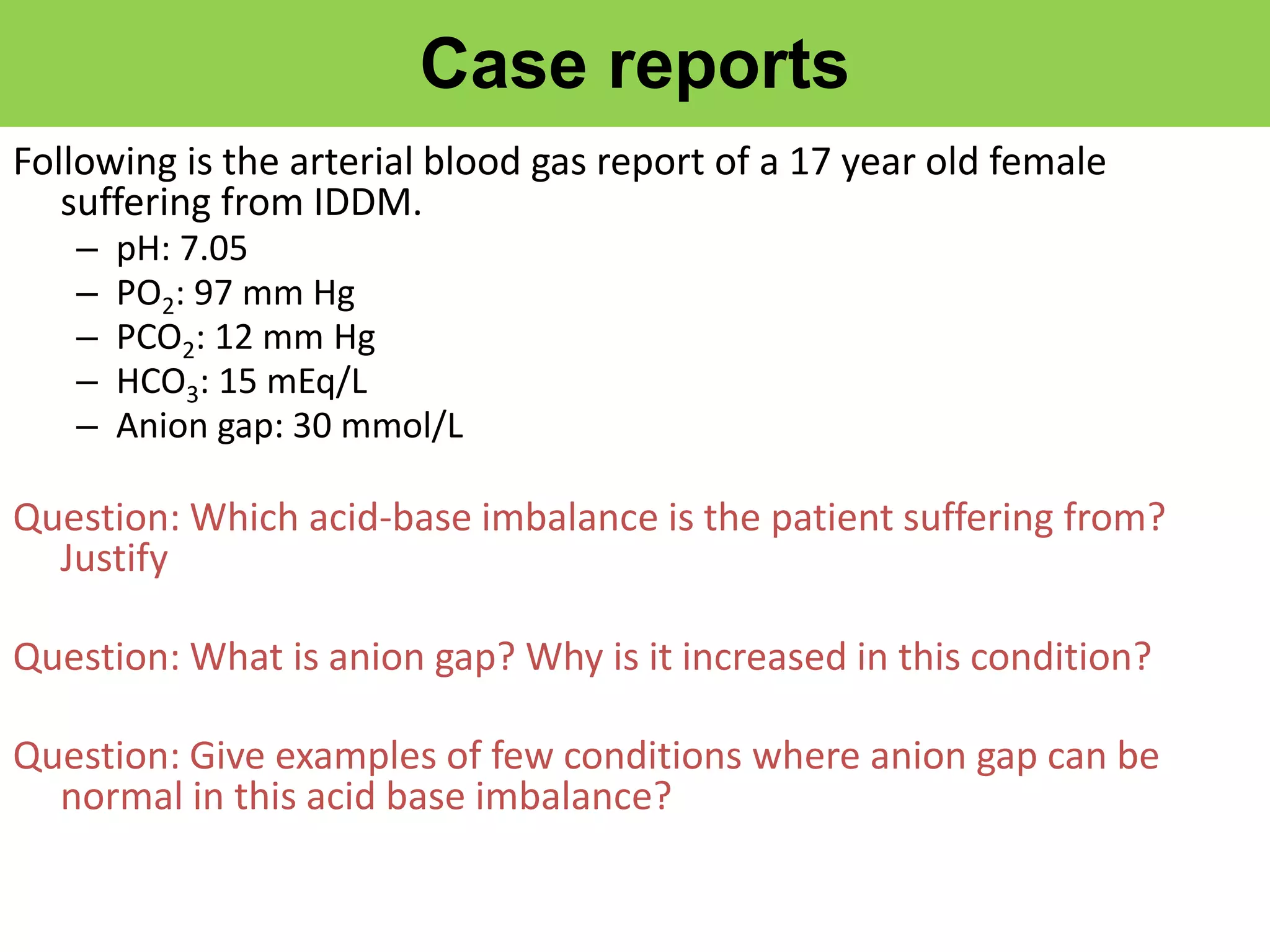
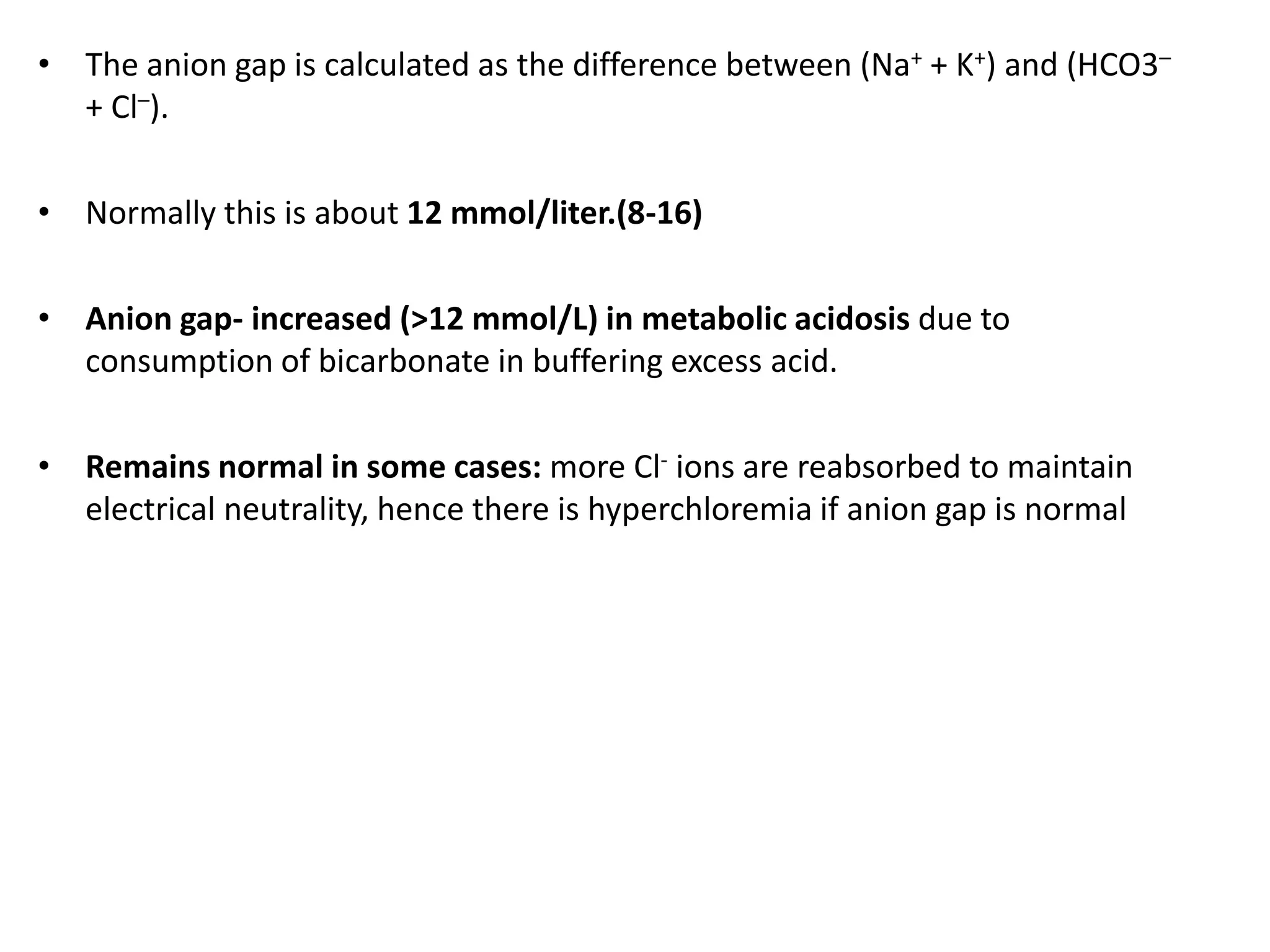
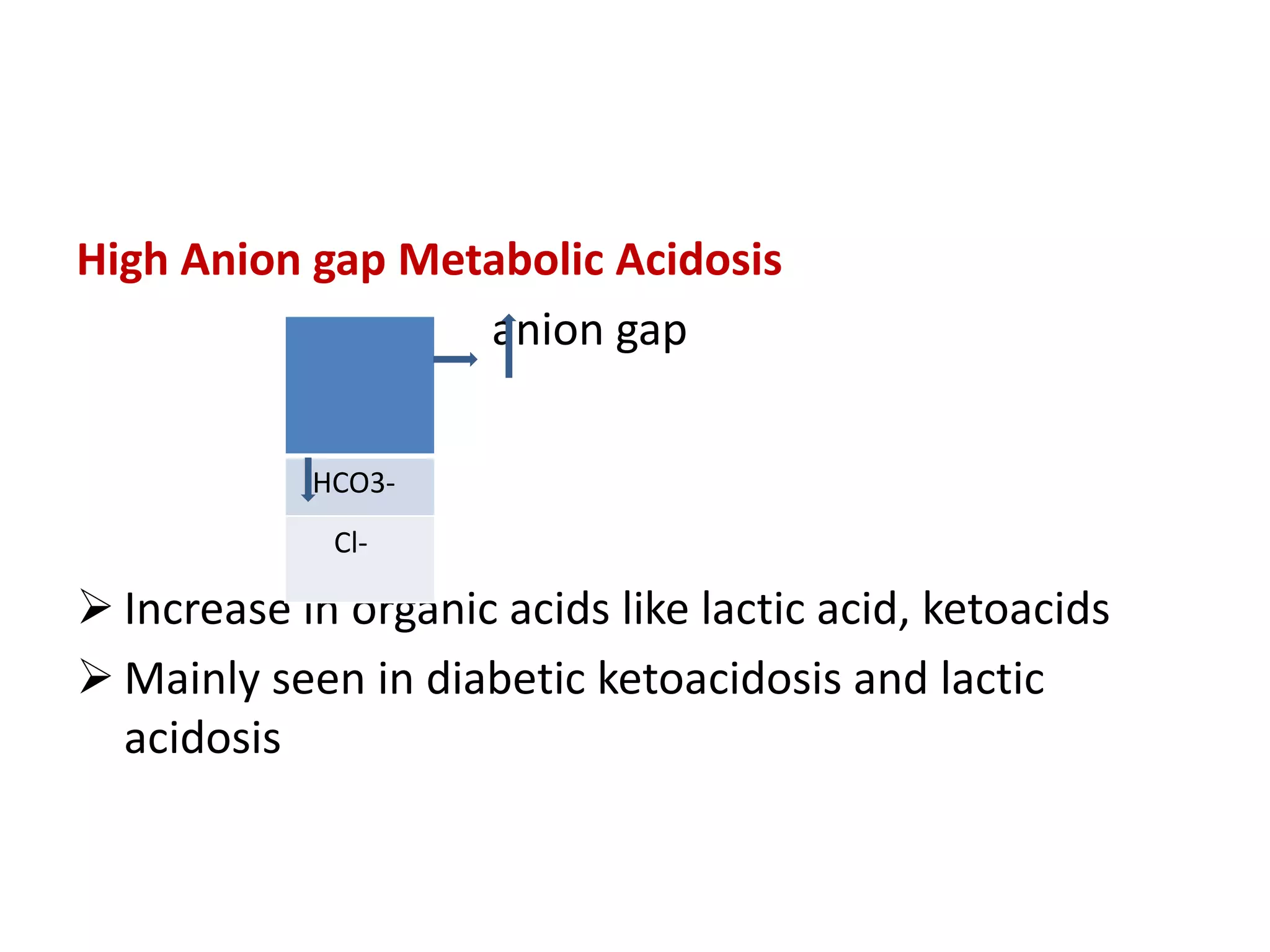
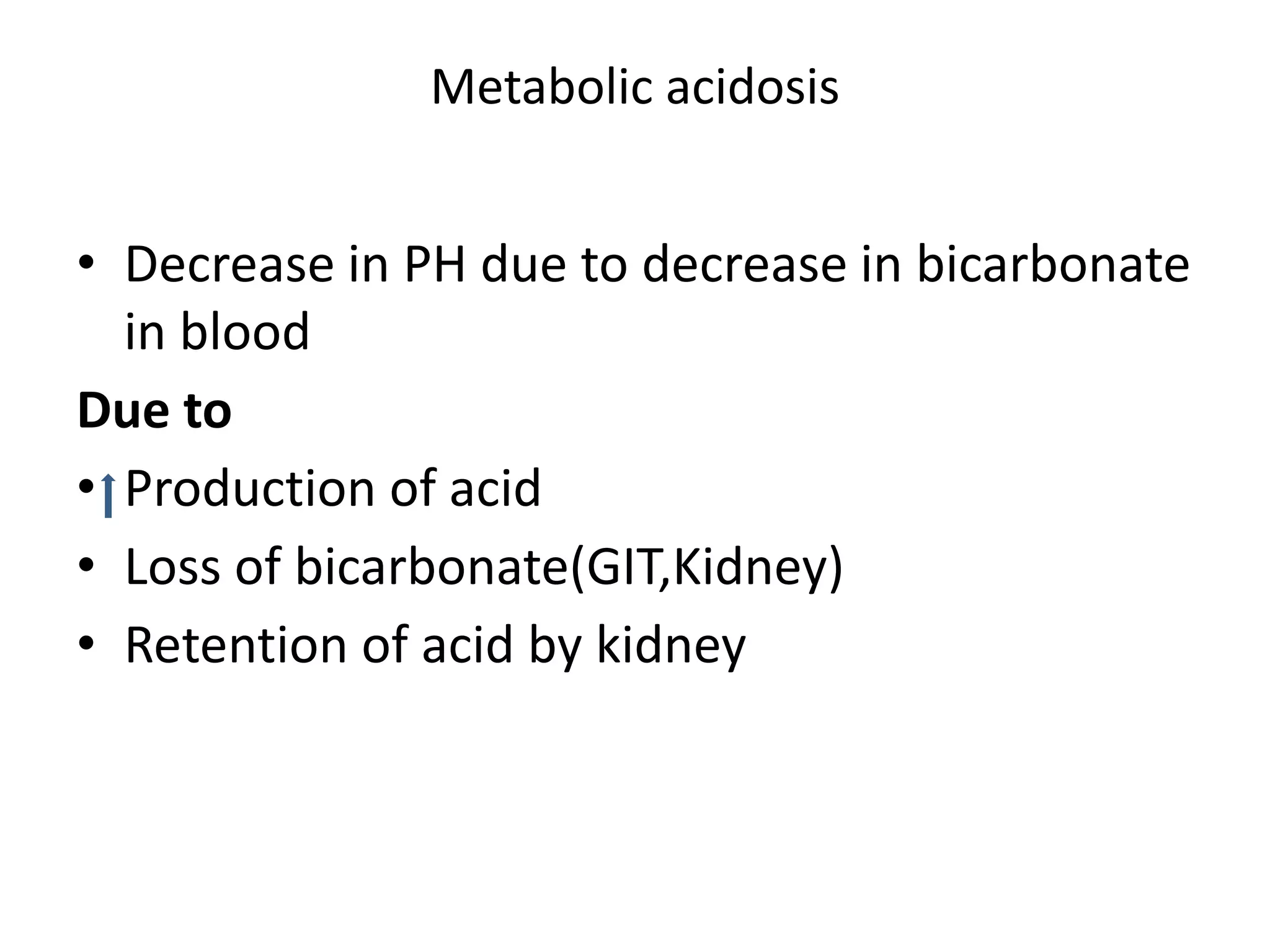
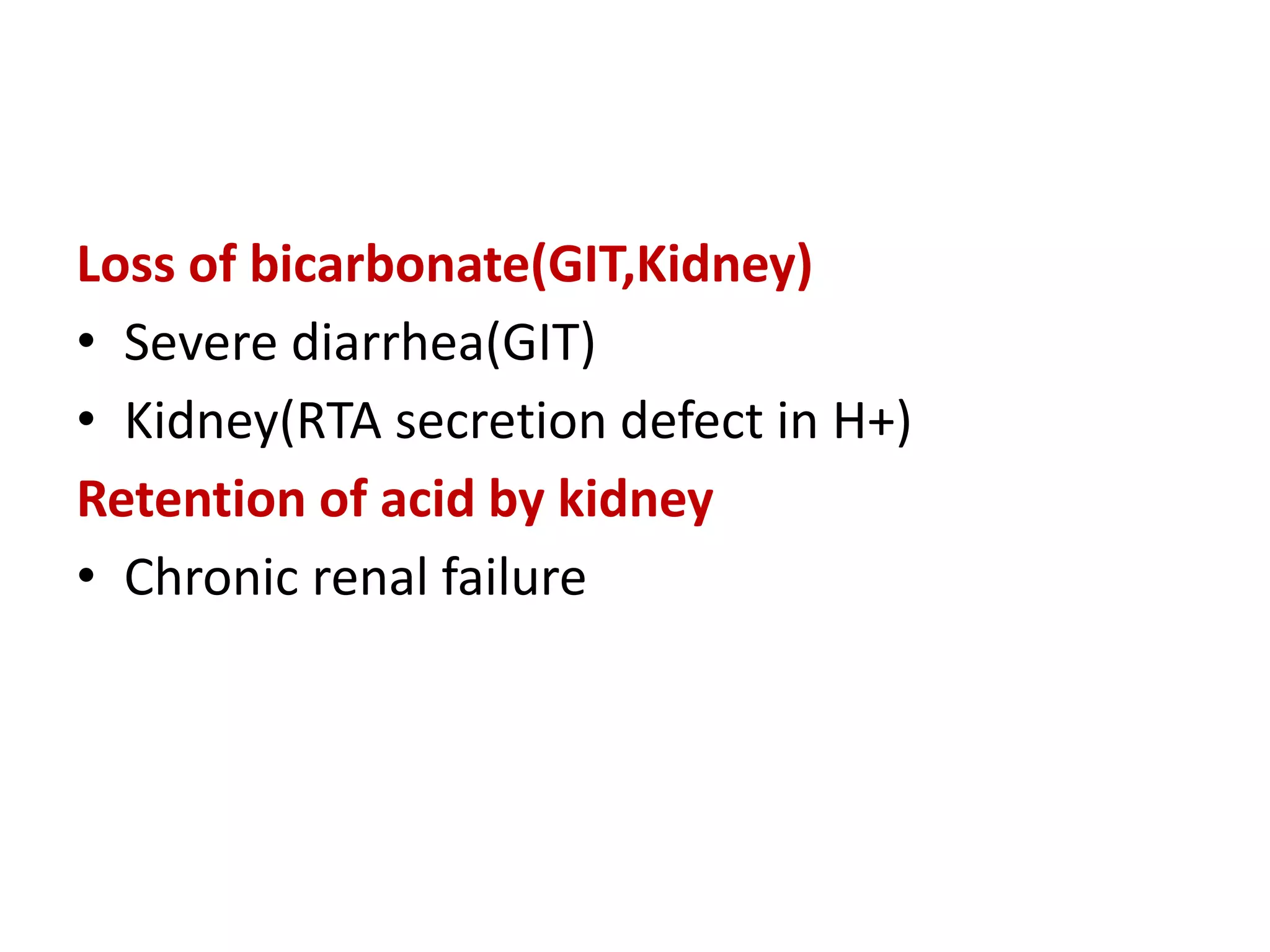
This document discusses acid-base balance and related disorders. It covers topics such as acids and bases, strong vs weak acids, blood buffers, and mechanisms of acid-base regulation including respiratory, renal, and buffering systems. The key points are:
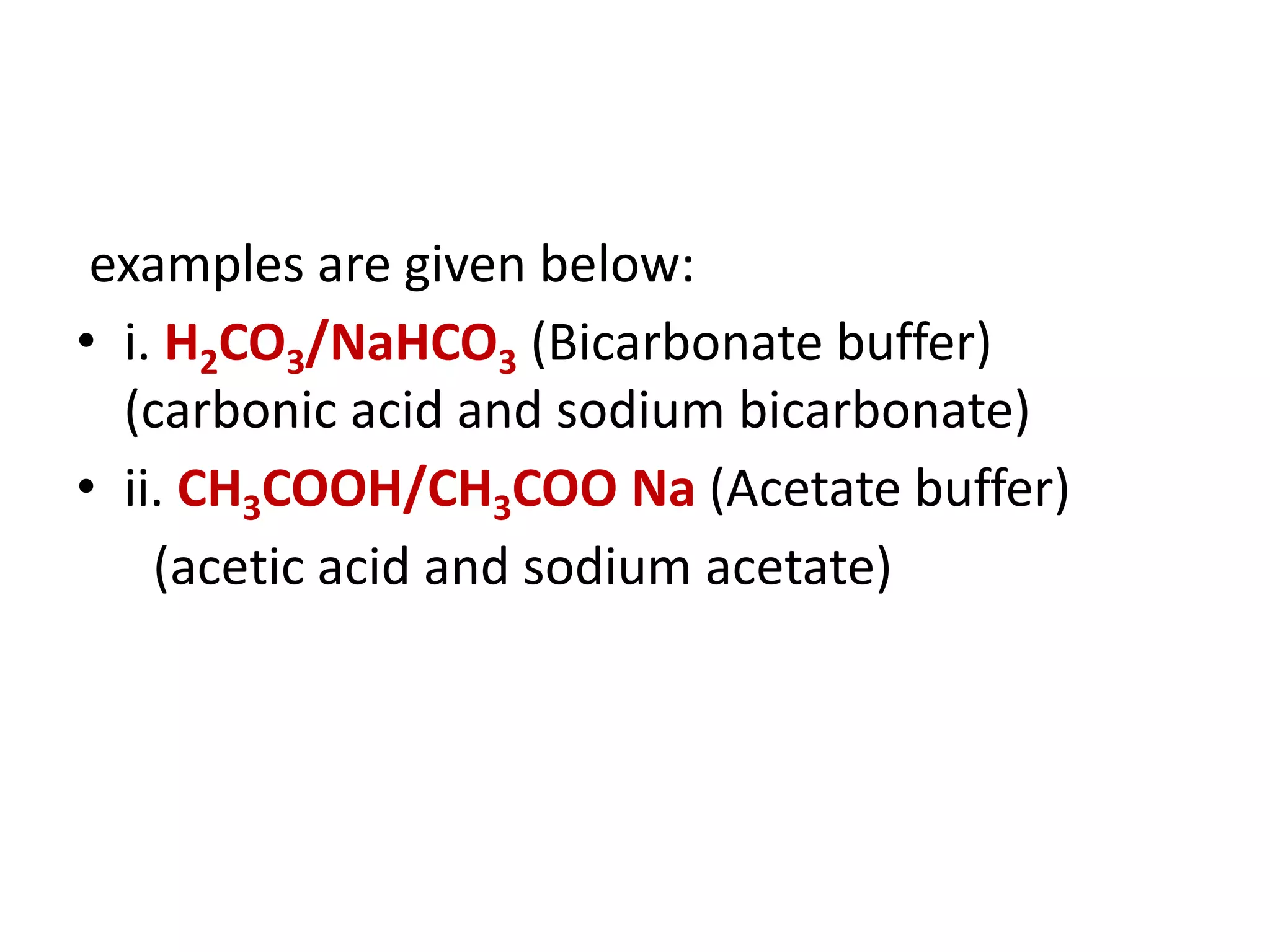
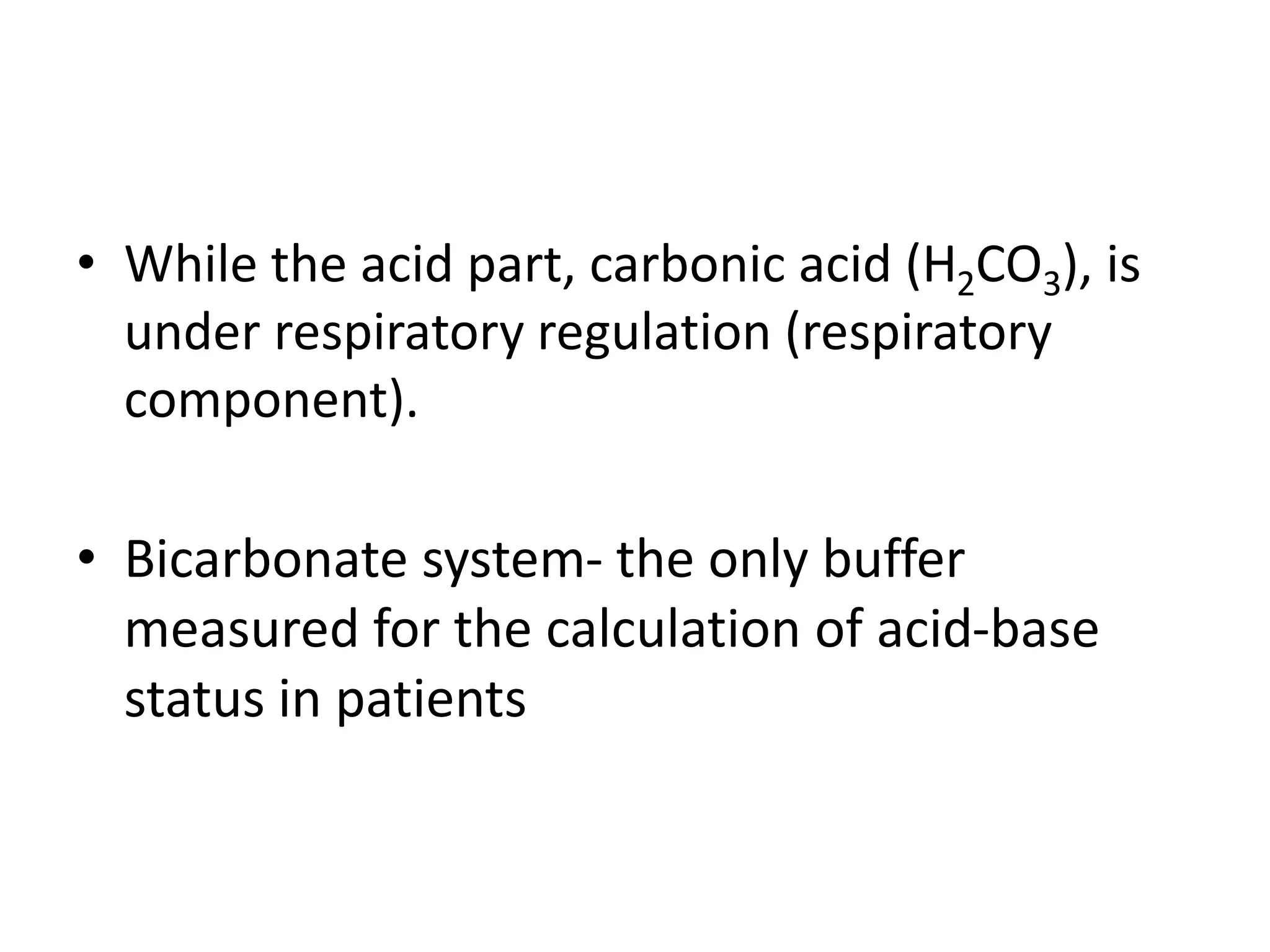
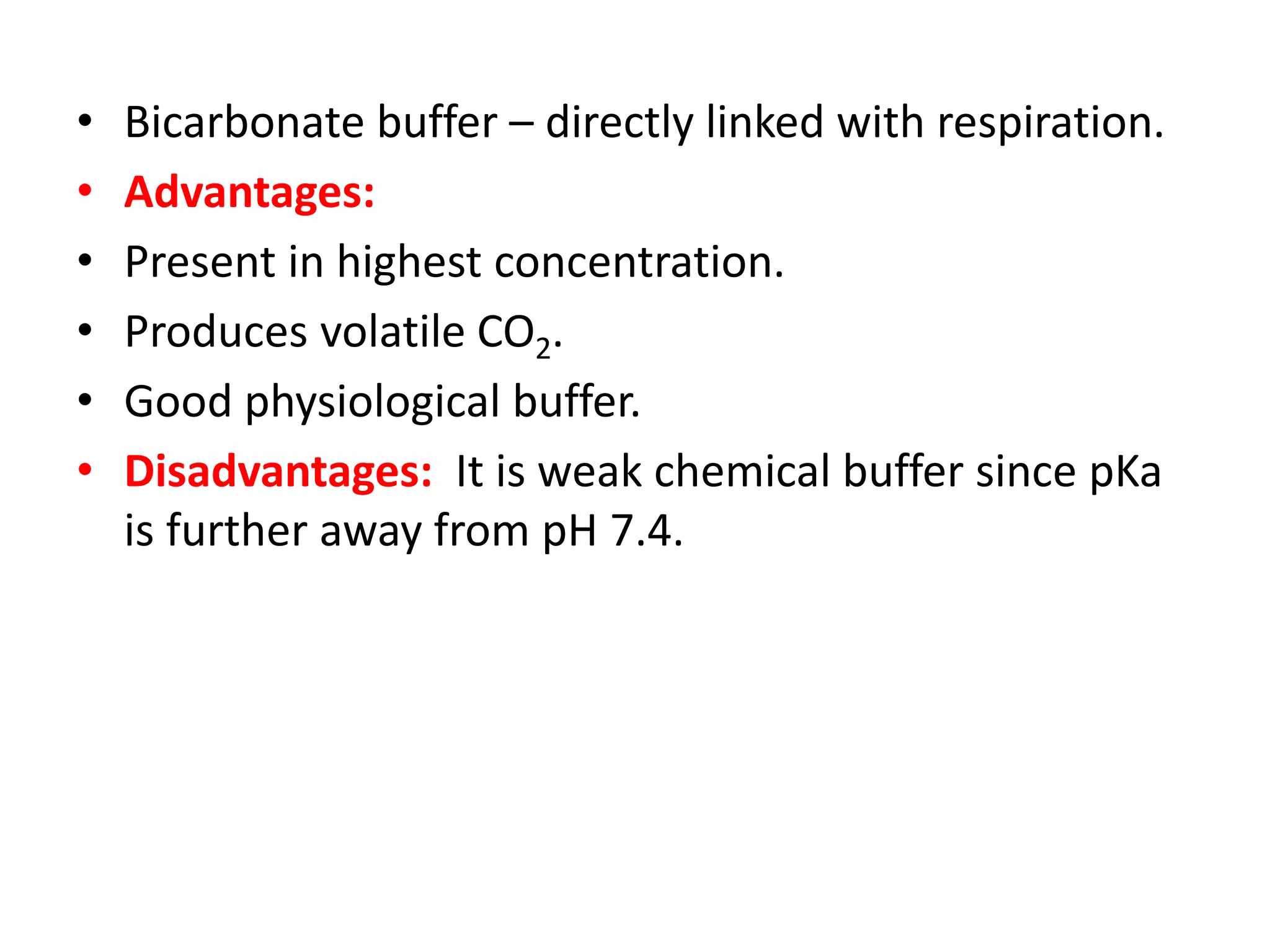
- The bicarbonate-carbonic acid buffer system is the most important blood buffer, accounting for 65% of buffering capacity. It is regulated by respiration and the kidneys.
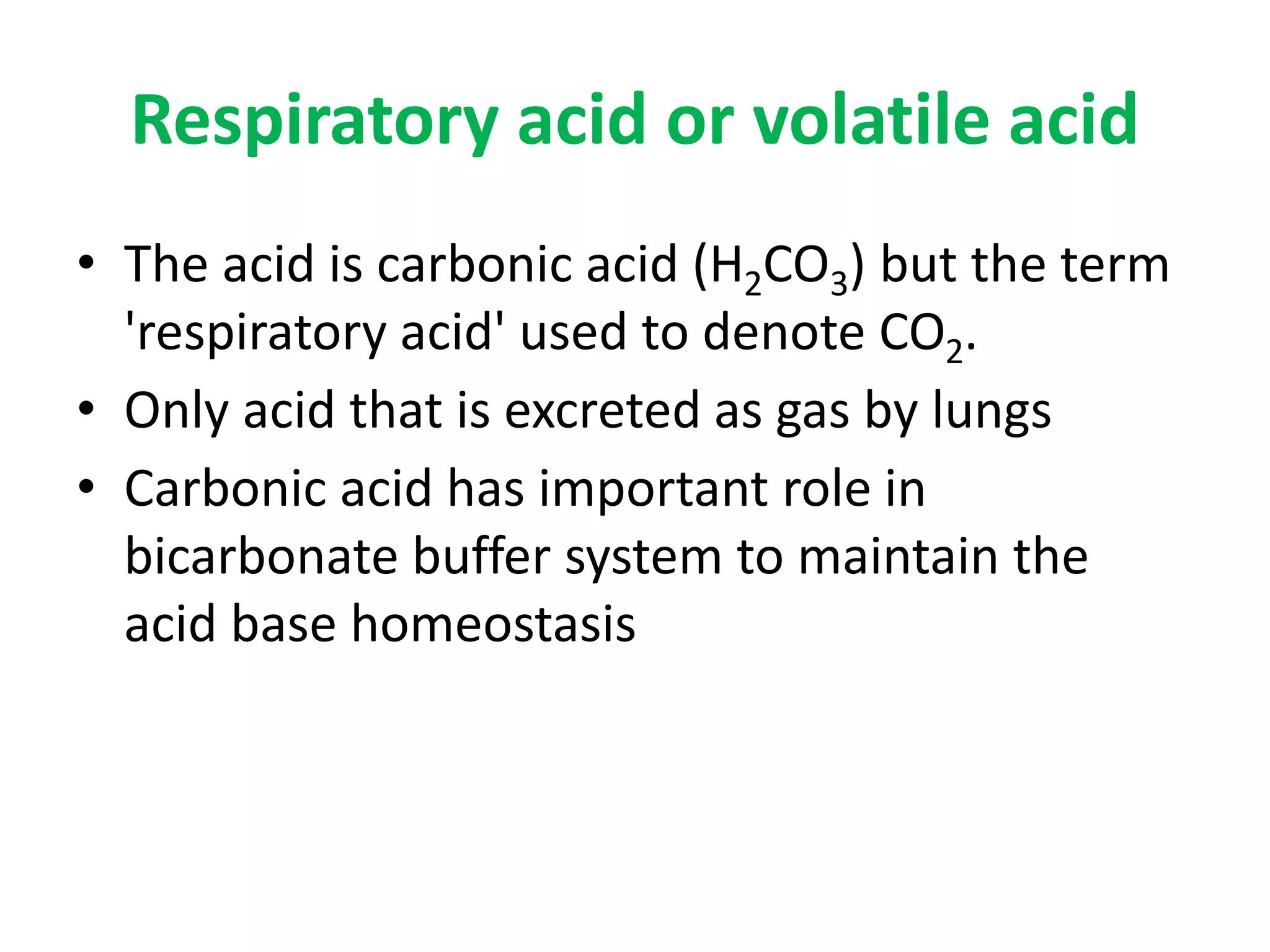
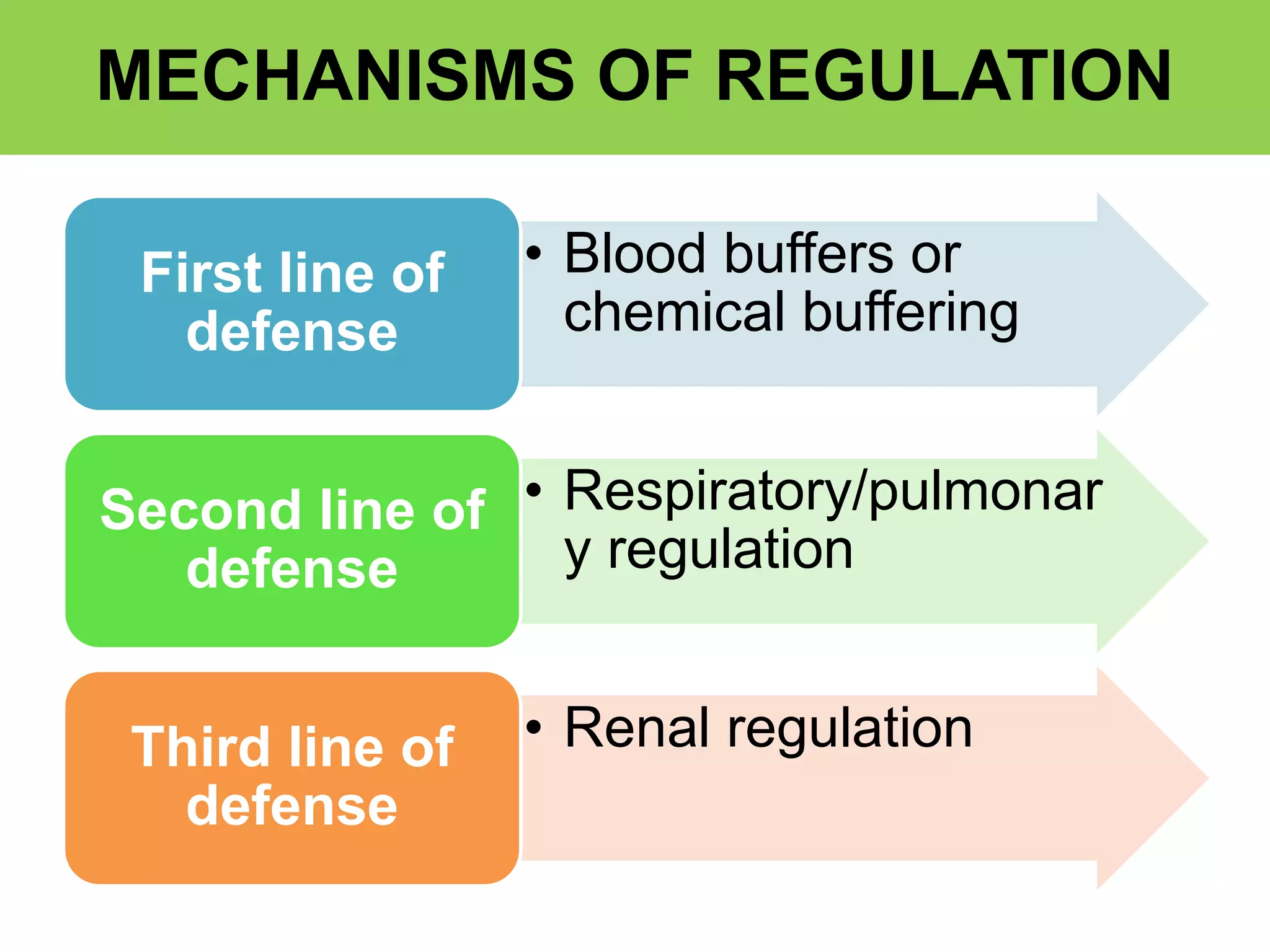
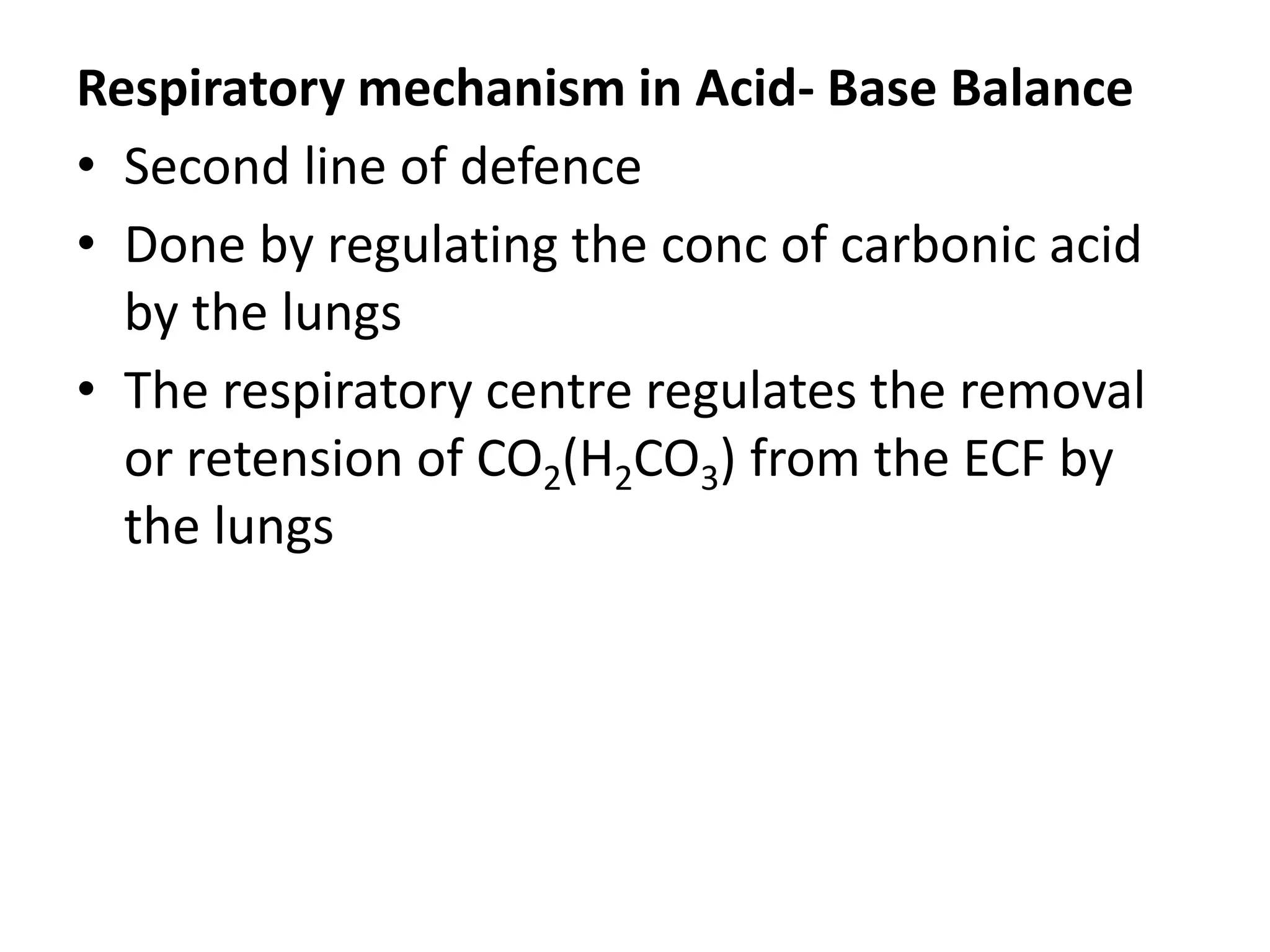
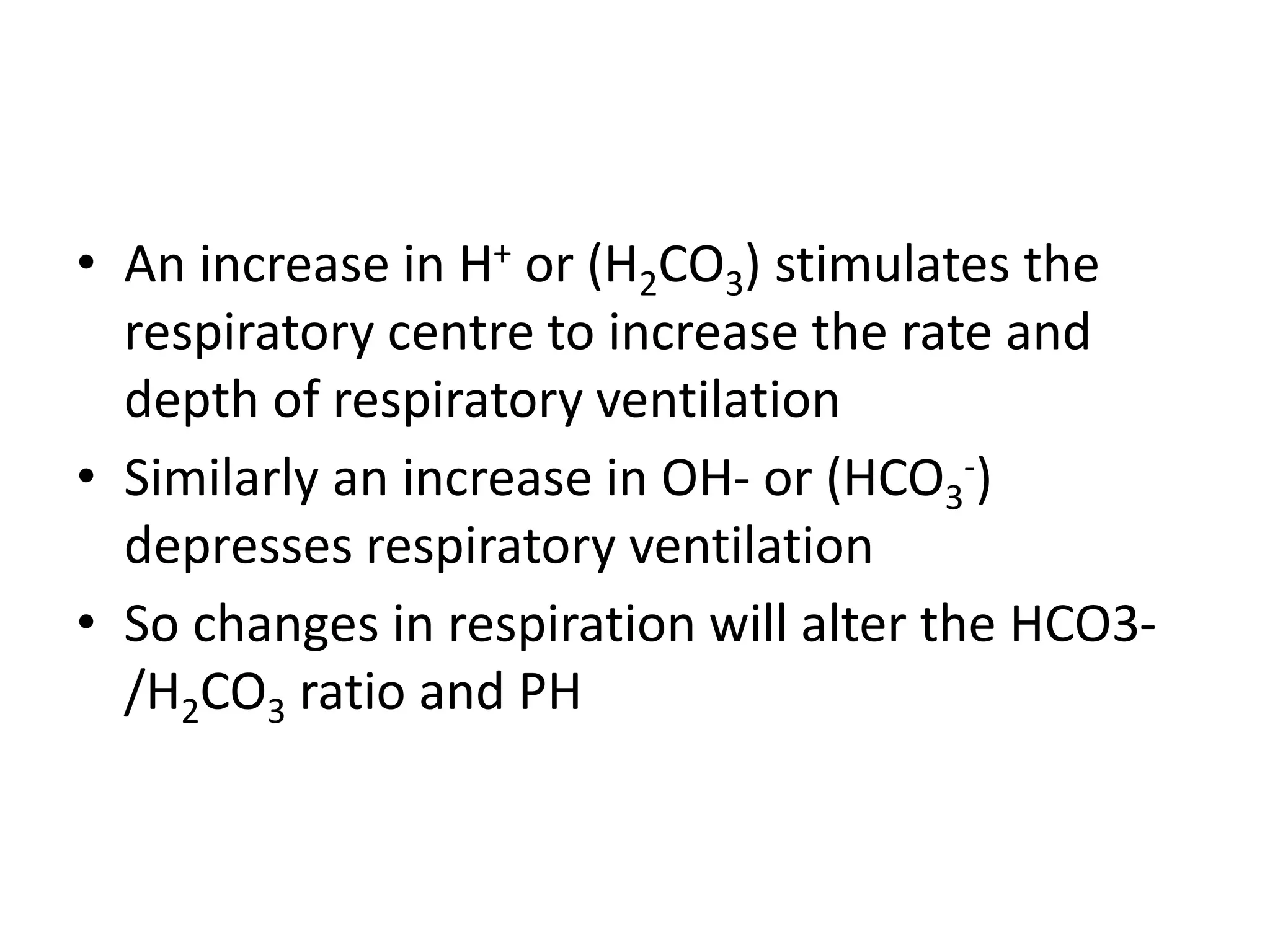
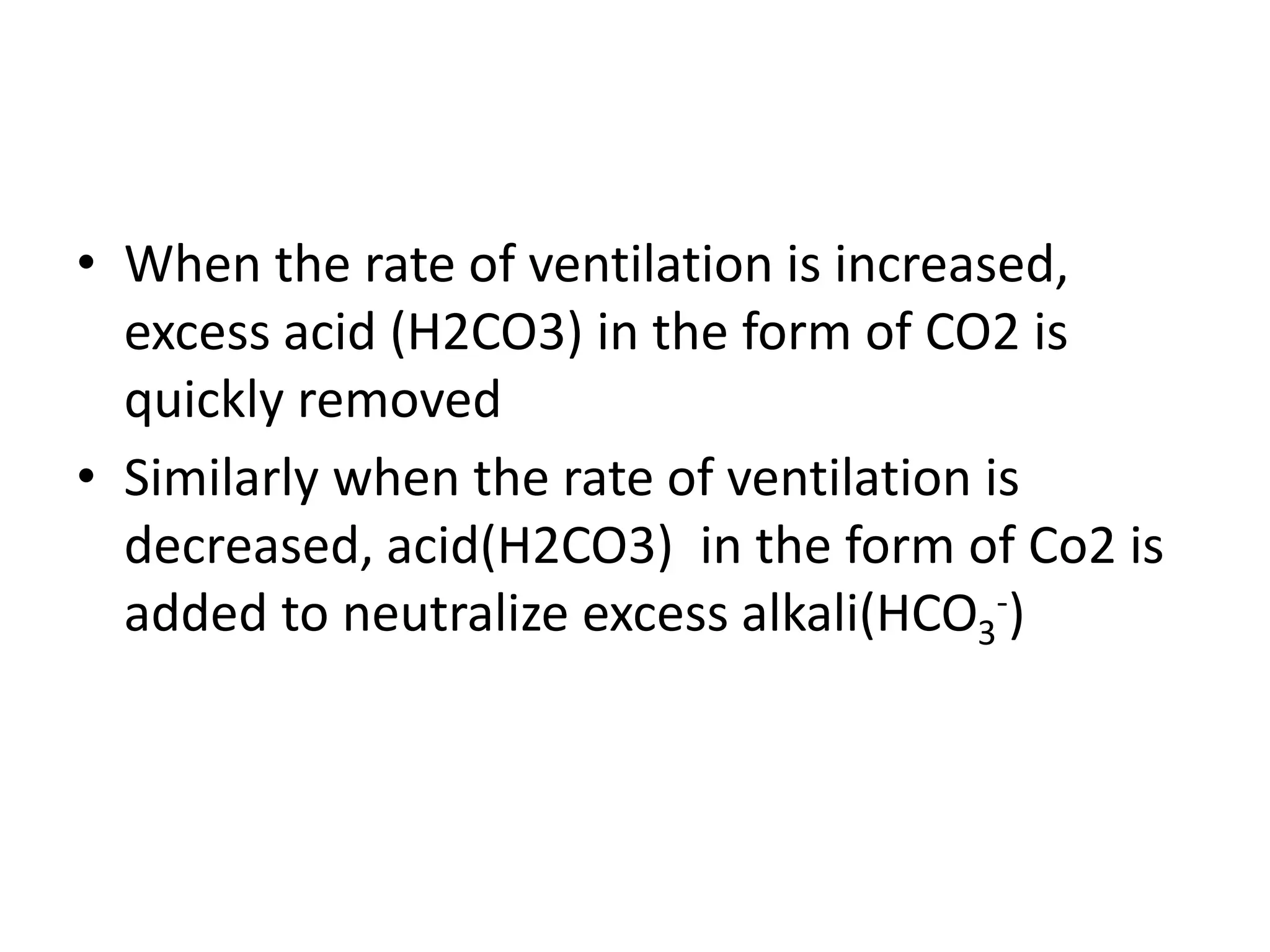
- Respiratory regulation is the second line of defense, controlling the concentration of carbonic acid by regulating respiration and CO2 levels.
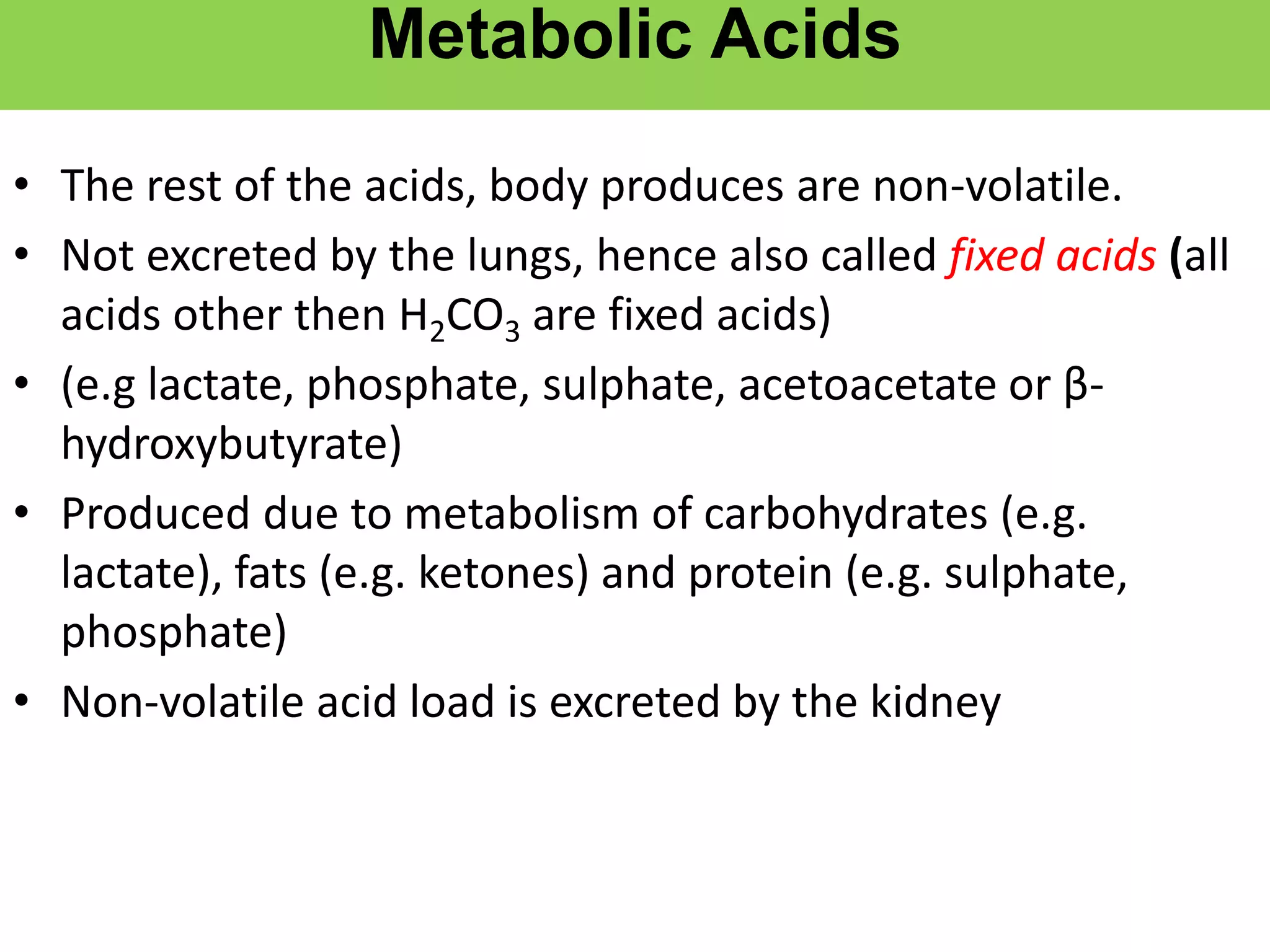
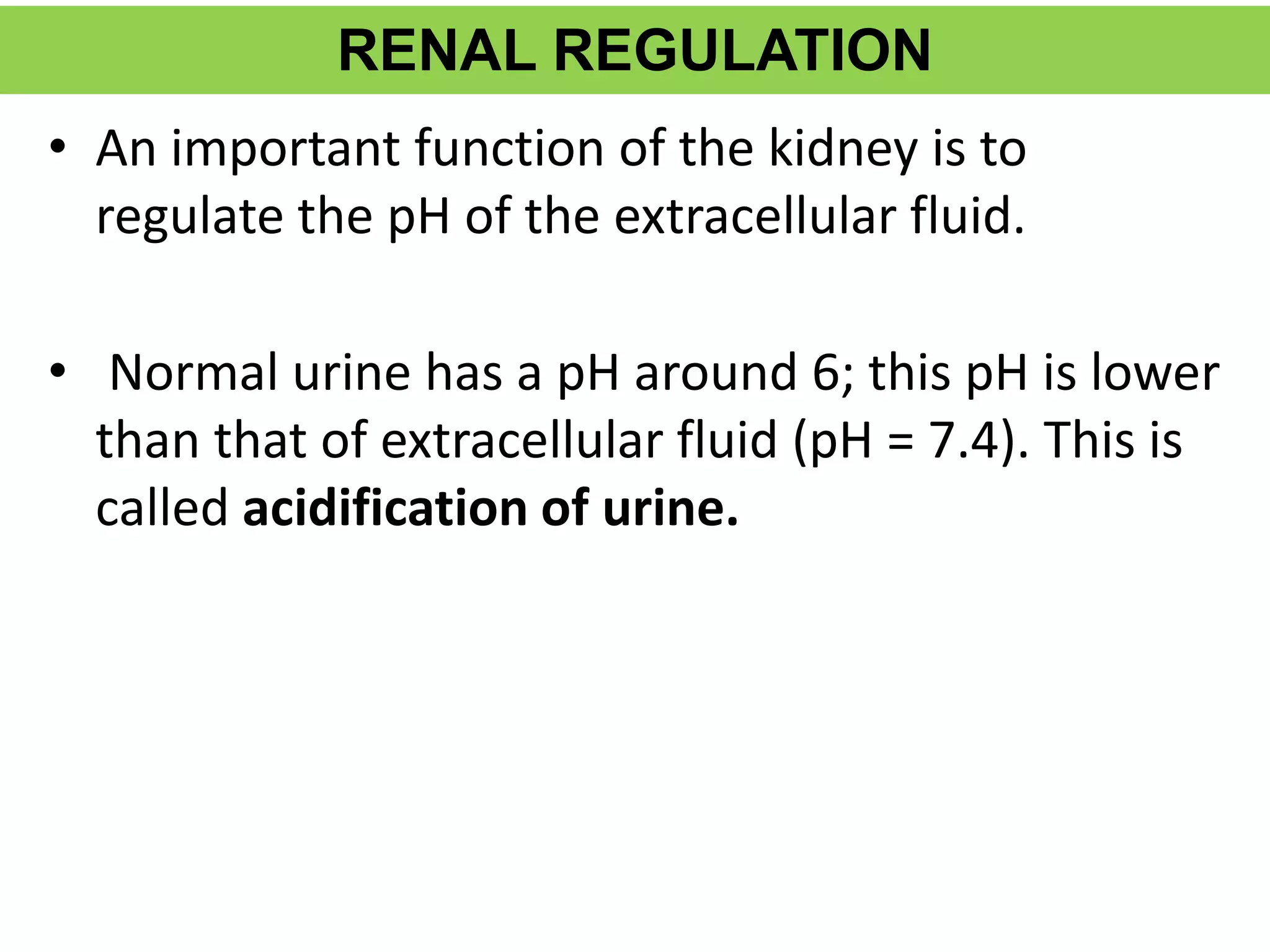
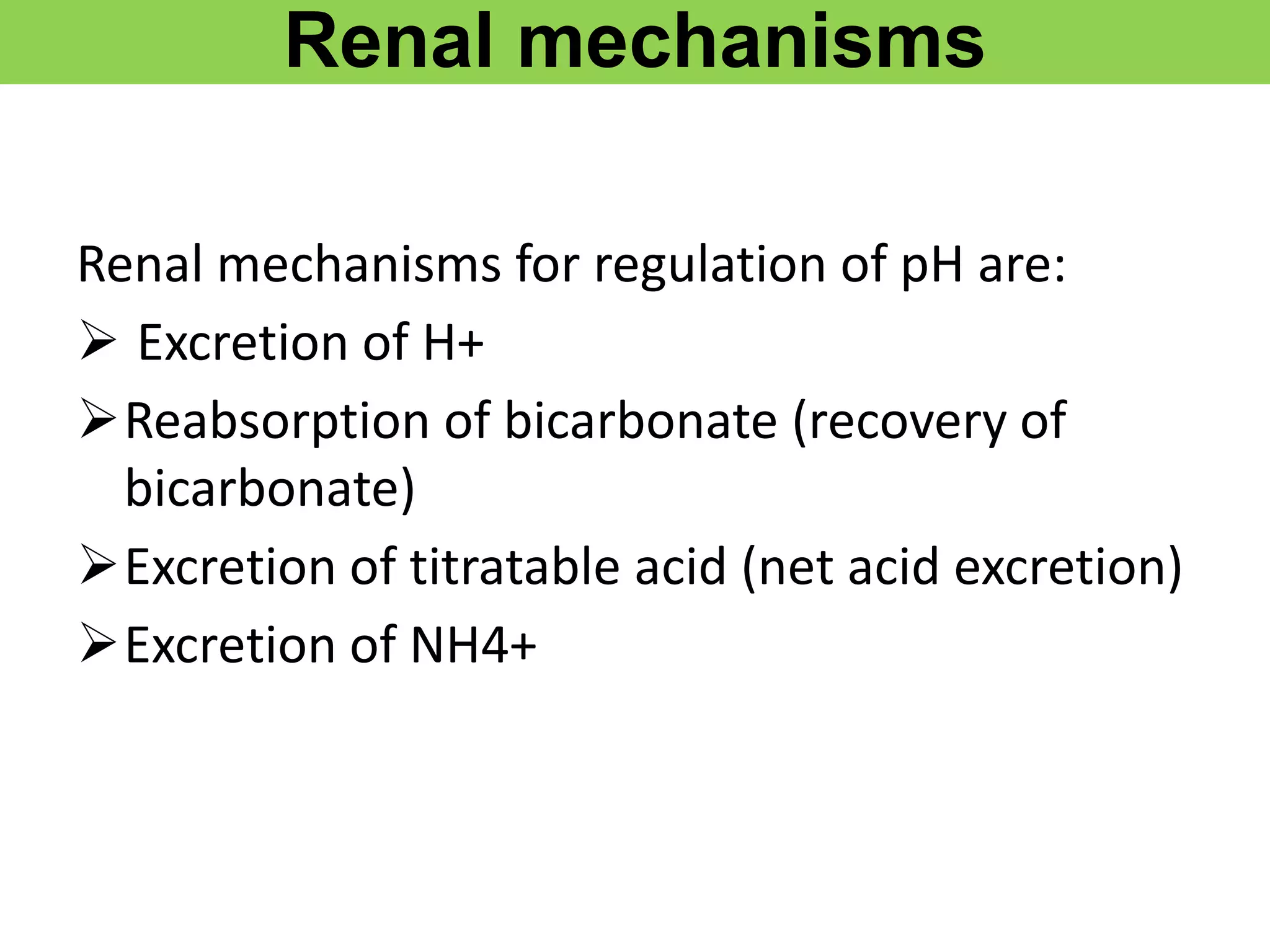
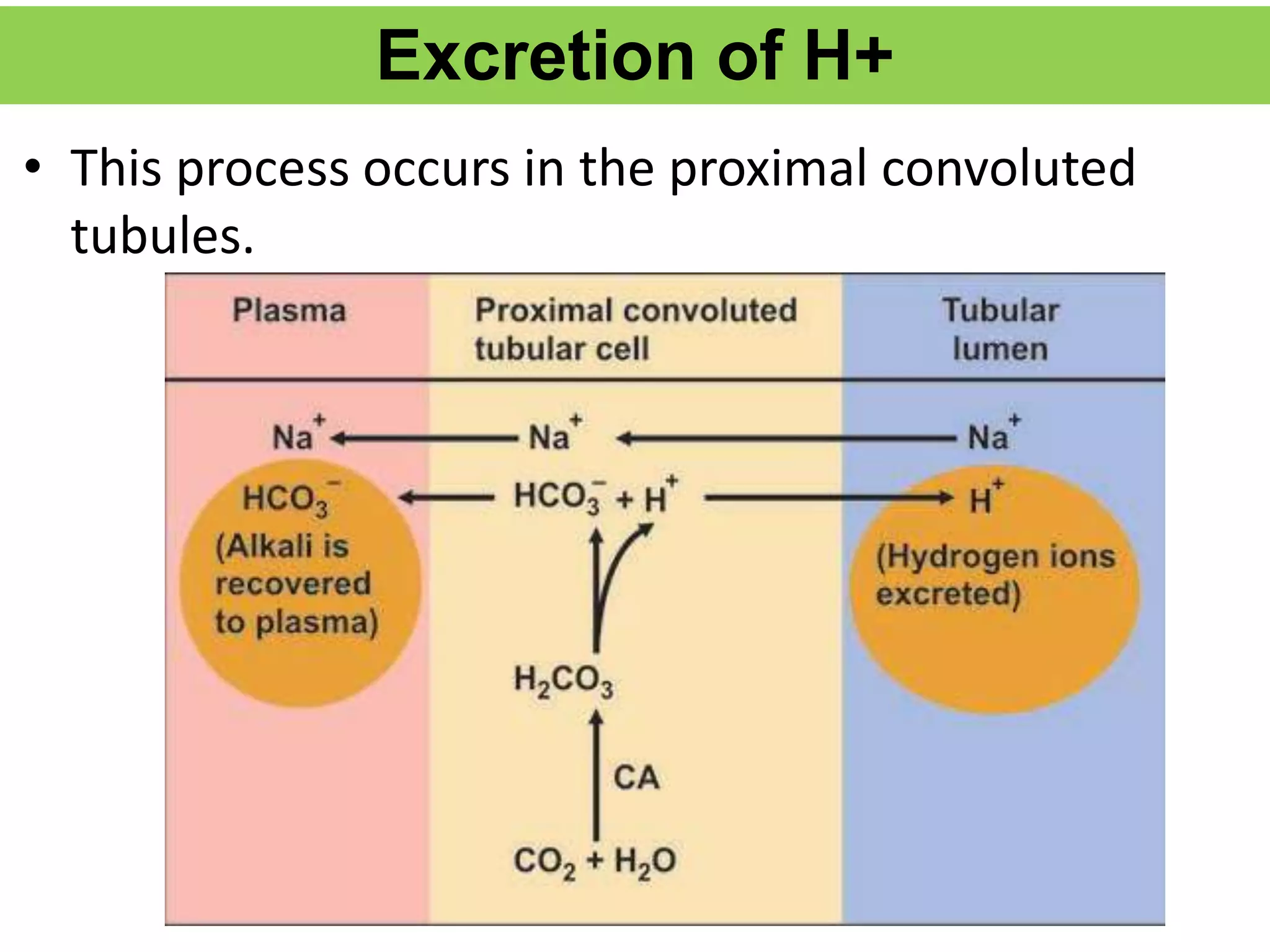
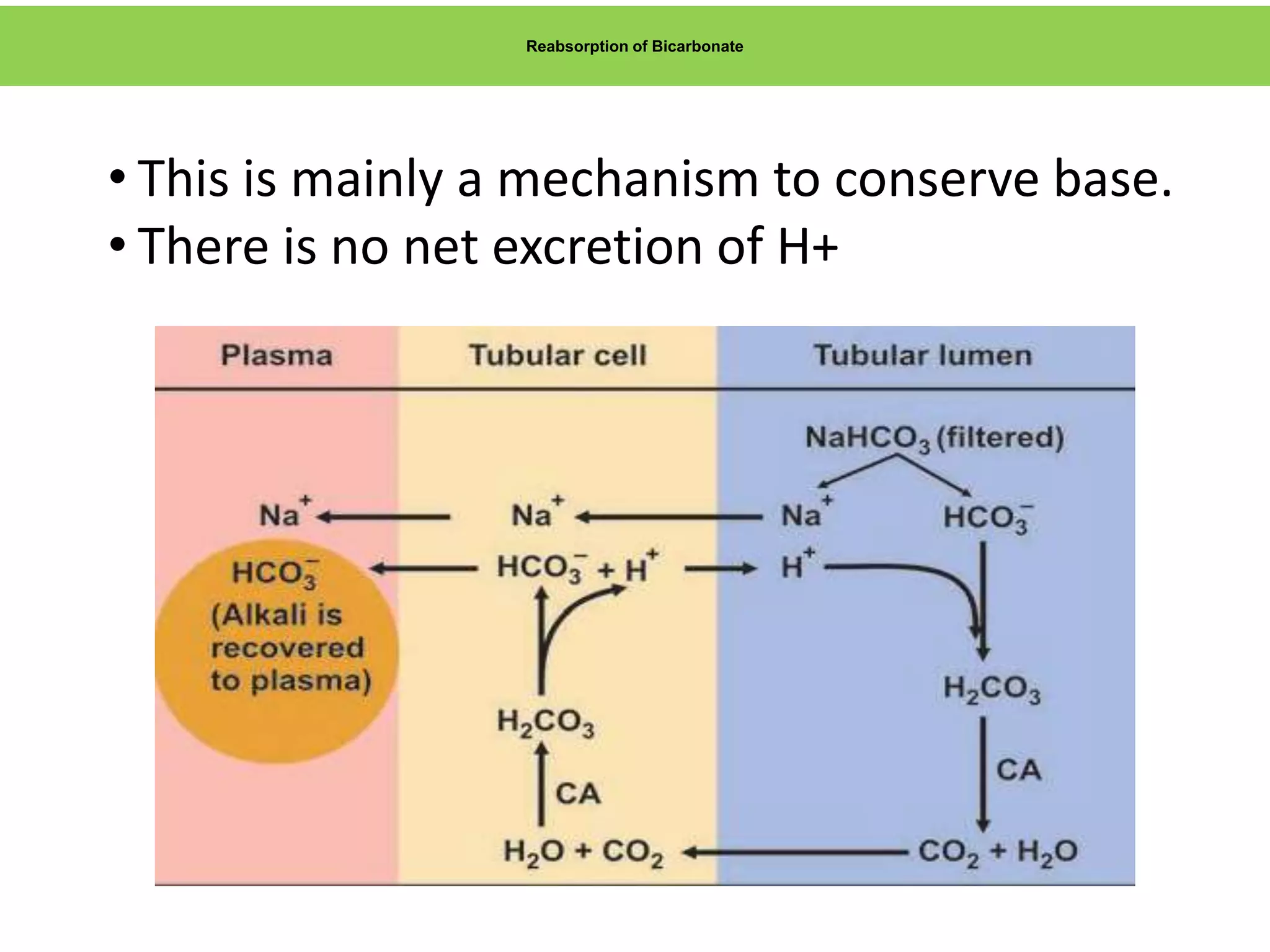
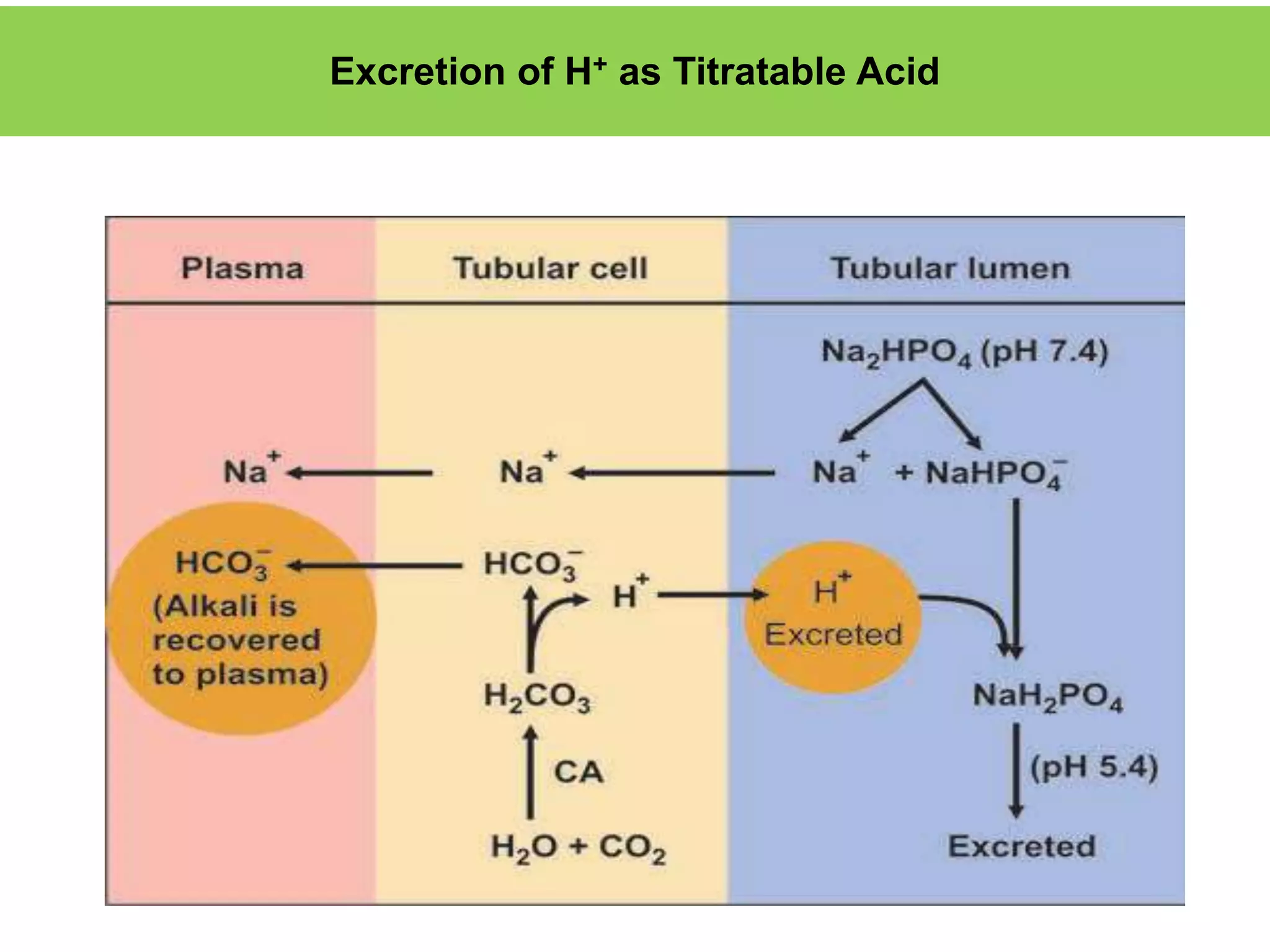
- Renal regulation is the third line of defense, maintaining acid-base balance by reabsorbing bicarbonate, ex


![Dissociation Constant
• The dissociation of an acid is a freely reversible reaction, at
equilibrium the ratio between dissociated and undissociated
particle is a constant. The dissociation constant (Ka) of an acid is
given by the formula:
Ka = [H+][A-]
[HA]
• [H+] concentration of hydrogen ions
• [A–] = the concentration of anions or conjugate base
• [HA] is the concentration of undissociated molecules.](https://image.slidesharecdn.com/acidbasebalancerelateddisorders-copy-220319062558/75/ACID-BASE-BALANCE-AND-RELATED-DISORDERS-Dr-M-PRIYANKA-6-2048.jpg)

![CONTD…
PH:
• Negative logarithm of hydrogen ion concentration – pH.
pH = –log [H+]
• pH value is inversely proportional to the acidity.
• Lower the pH, higher the acidity or hydrogen ion
concentration while higher the pH, the acidity is lower.
• pH 7 indicates the neutral pH.](https://image.slidesharecdn.com/acidbasebalancerelateddisorders-copy-220319062558/75/ACID-BASE-BALANCE-AND-RELATED-DISORDERS-Dr-M-PRIYANKA-8-2048.jpg)









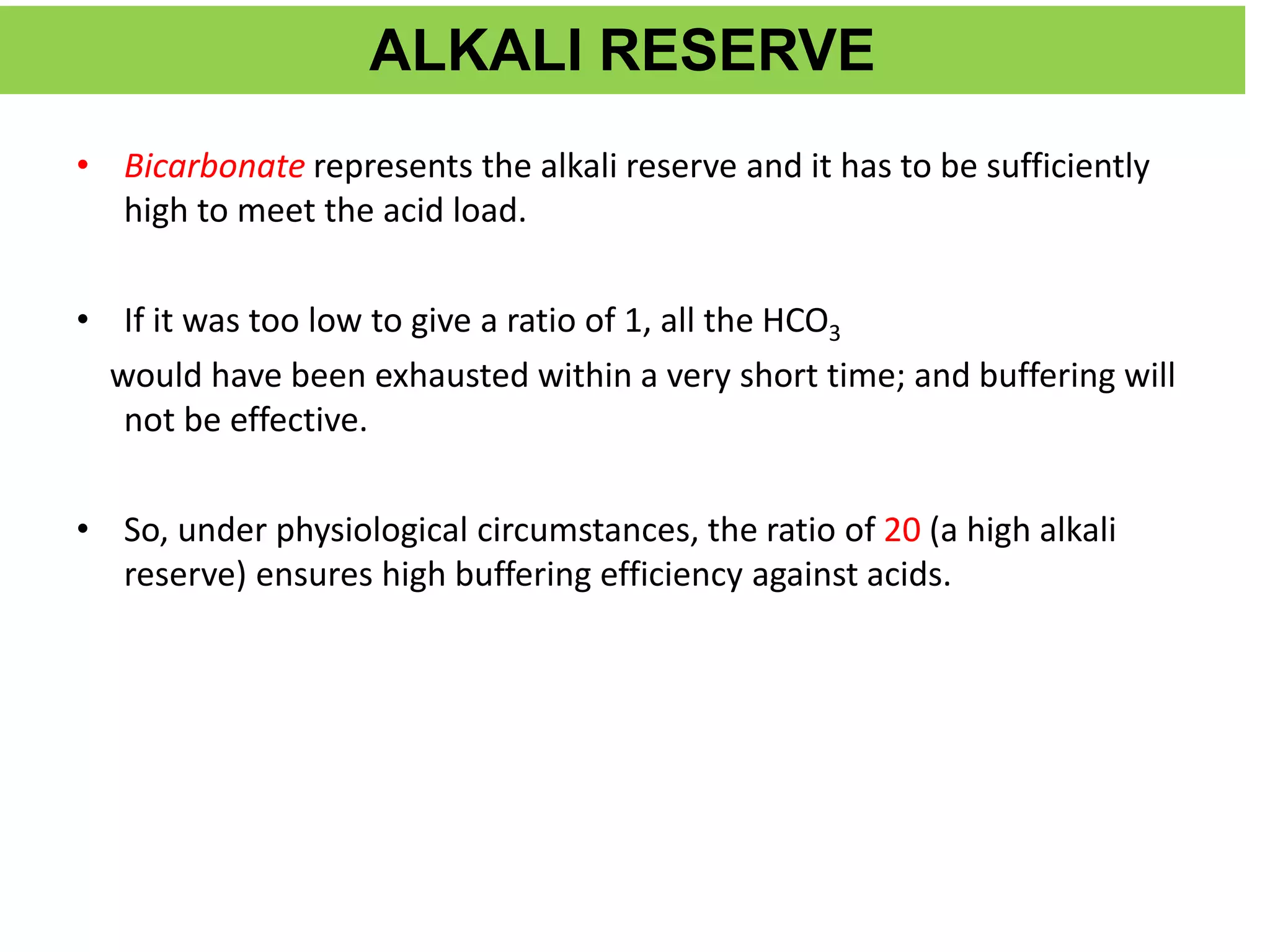
![• The normal bicarbonate level of plasma is 24
mmol/liter
• Normal pCO2 of arterial blood is 40mm of Hg
• Normal H2CO3 is 1.2mmol/L
• PKa of carbonic acid is 6.1
PH = Pka +log [HCO3
-]
H2CO3
• 7.4= 6.1 +log24 =20
1.2
Hence the ratio of HCO3 to H2CO3 at PH 7.4 is 20](https://image.slidesharecdn.com/acidbasebalancerelateddisorders-copy-220319062558/75/ACID-BASE-BALANCE-AND-RELATED-DISORDERS-Dr-M-PRIYANKA-26-2048.jpg)
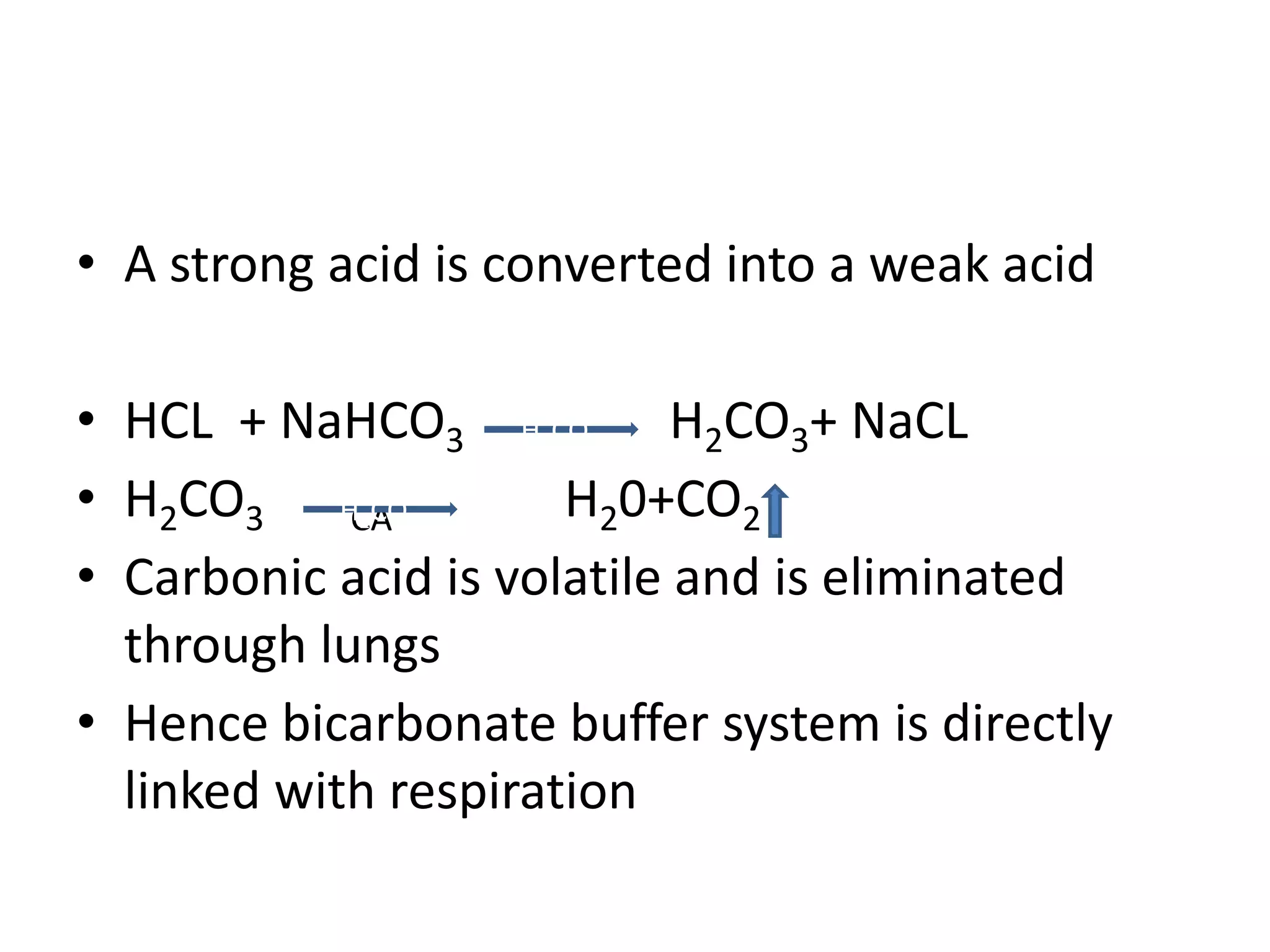
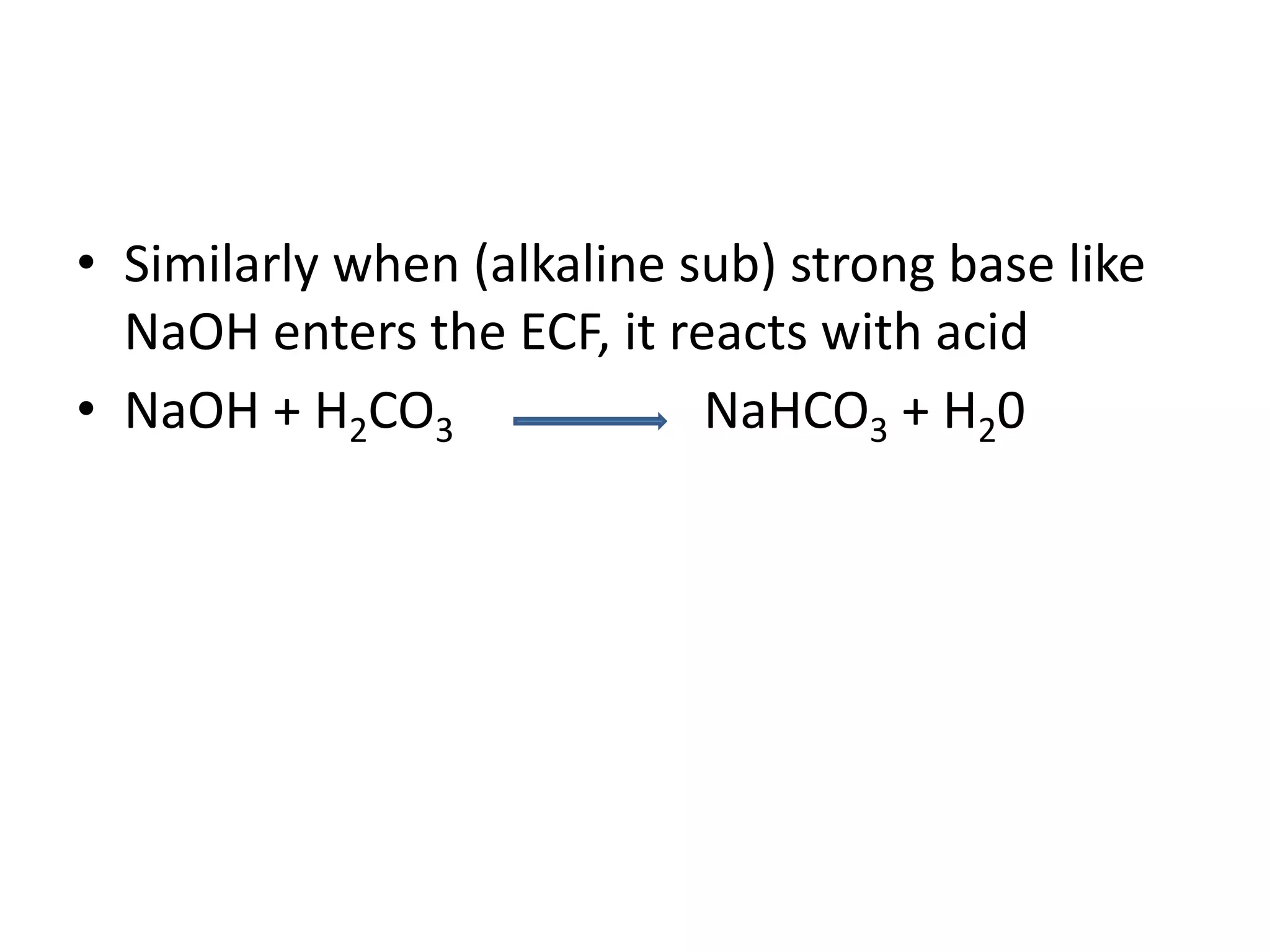


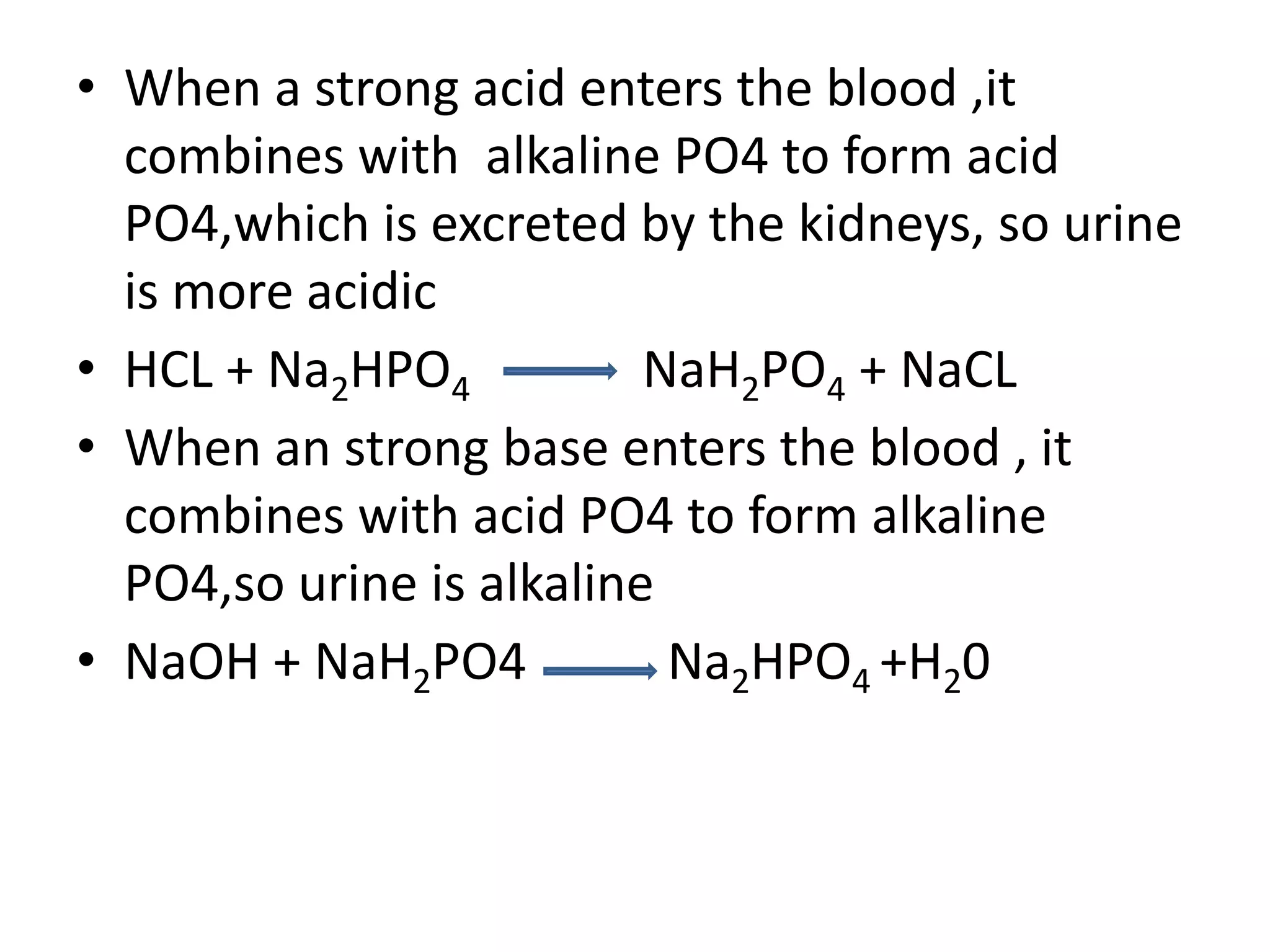




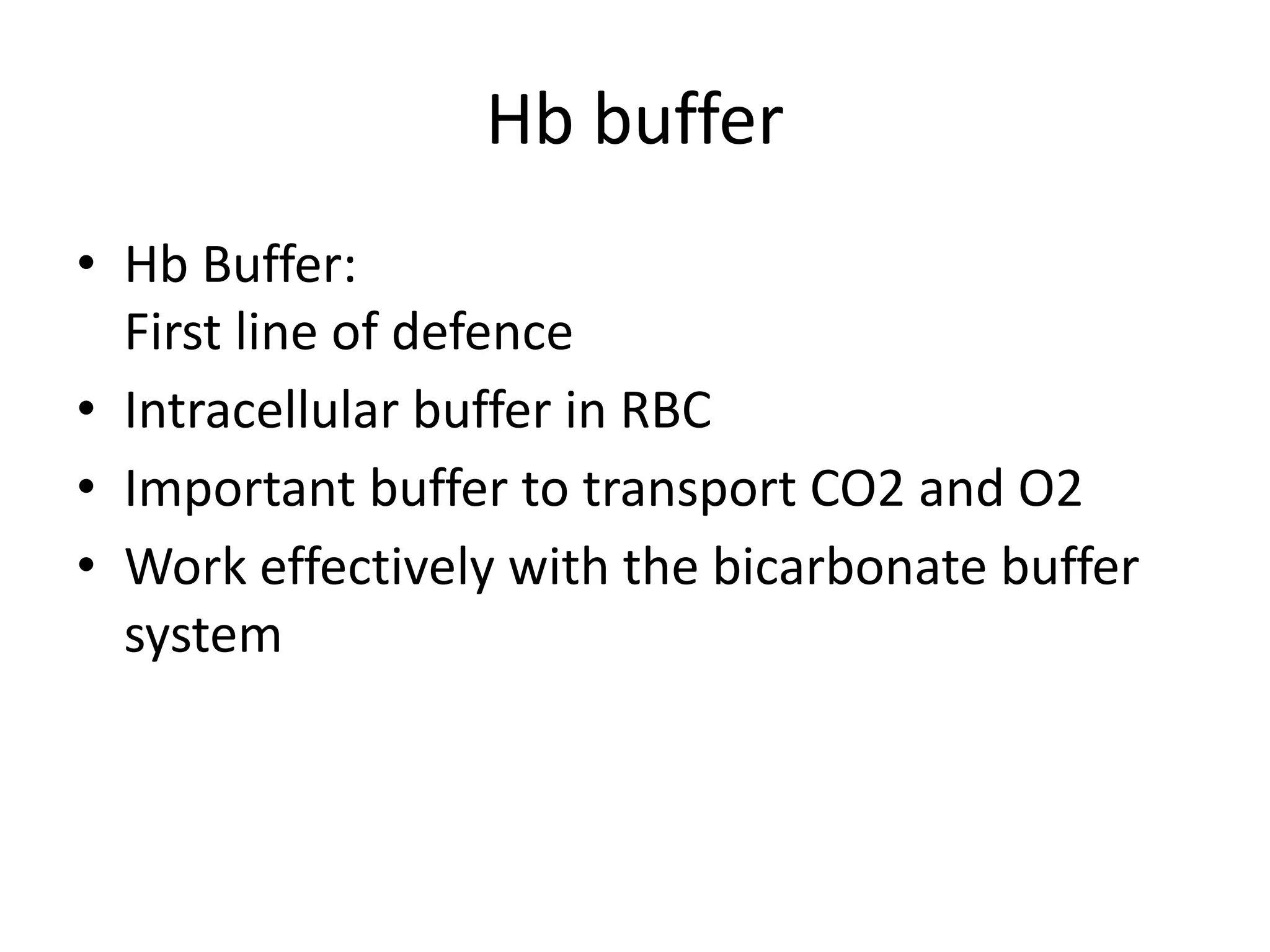
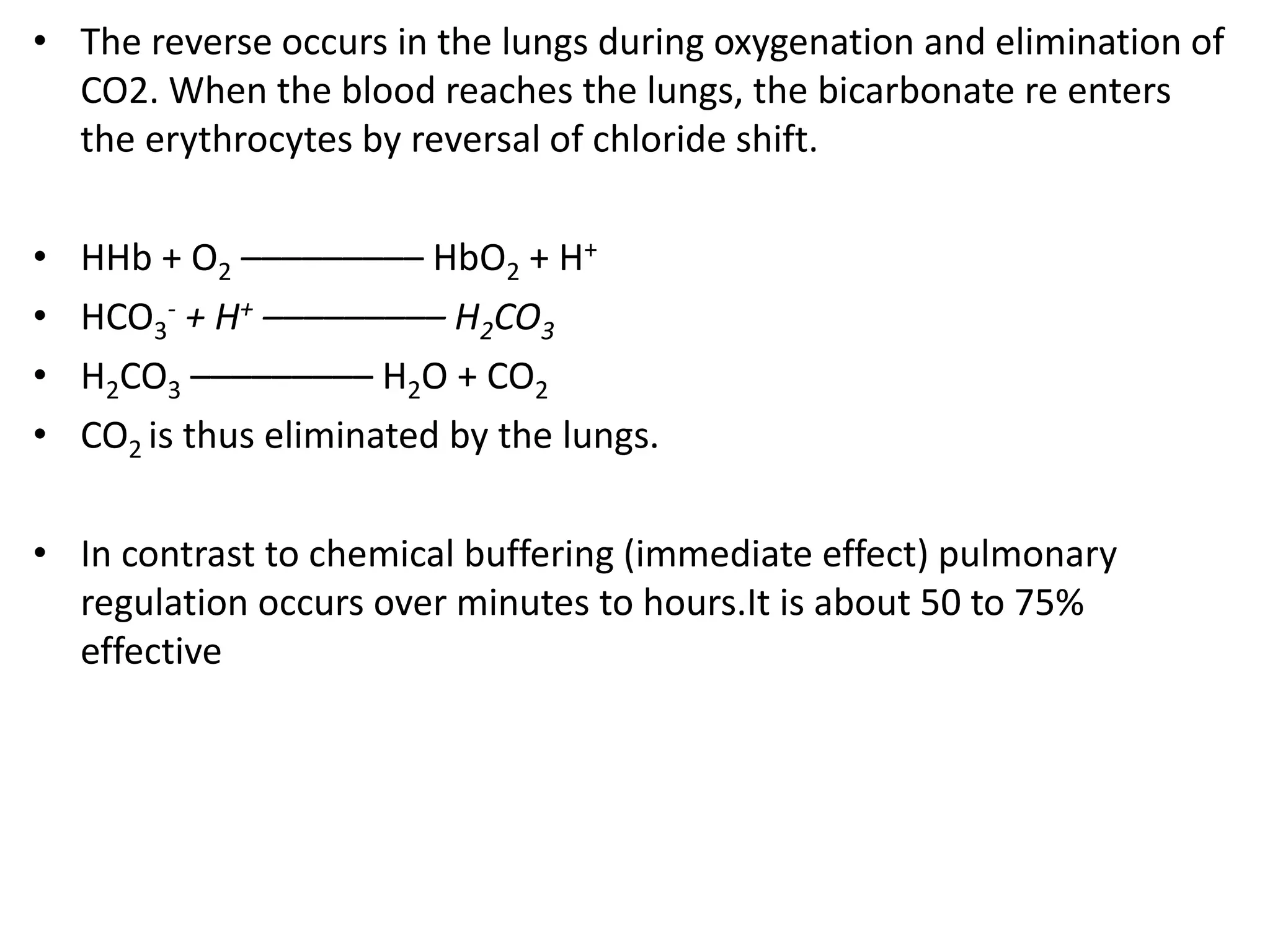
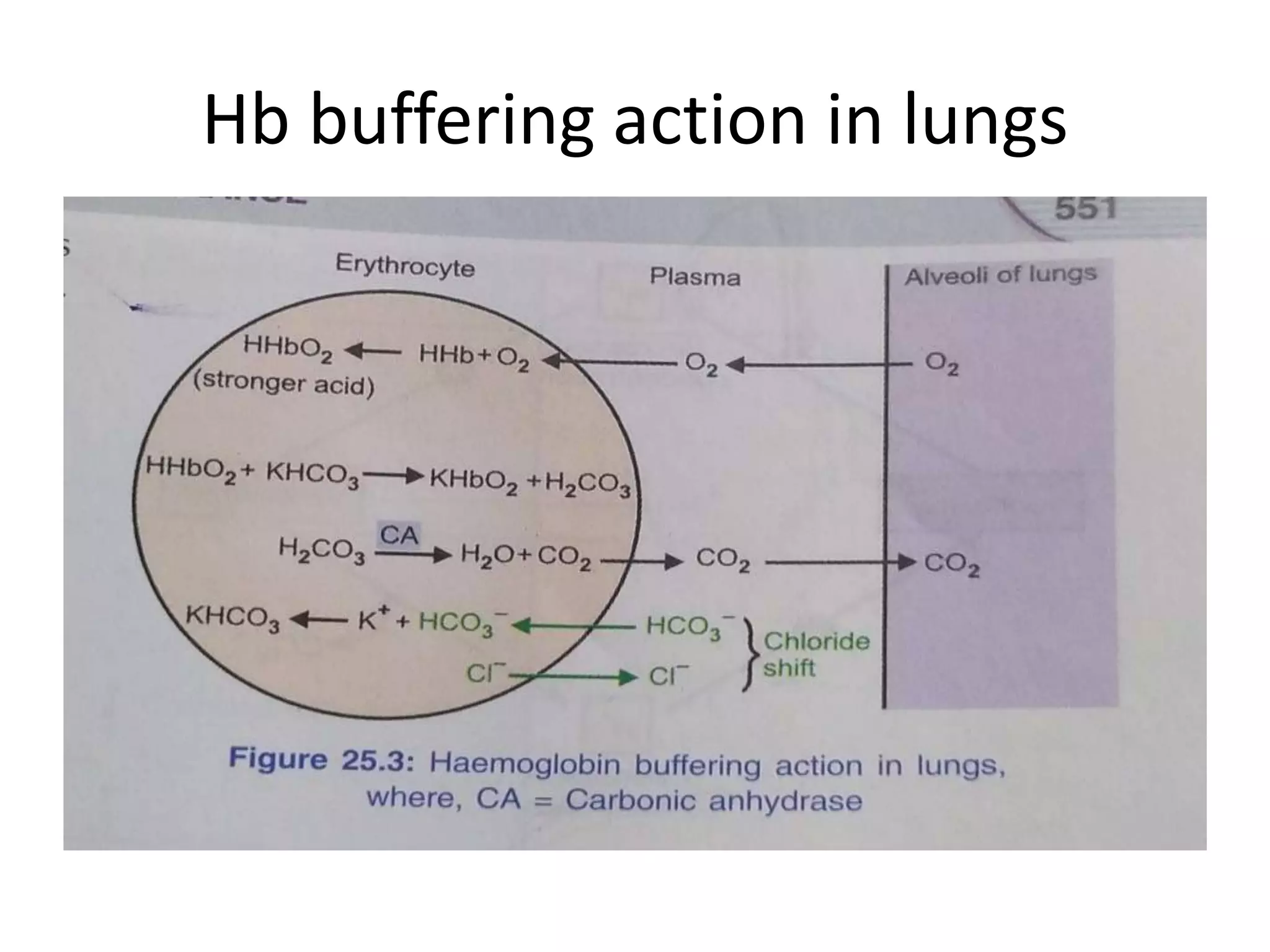




























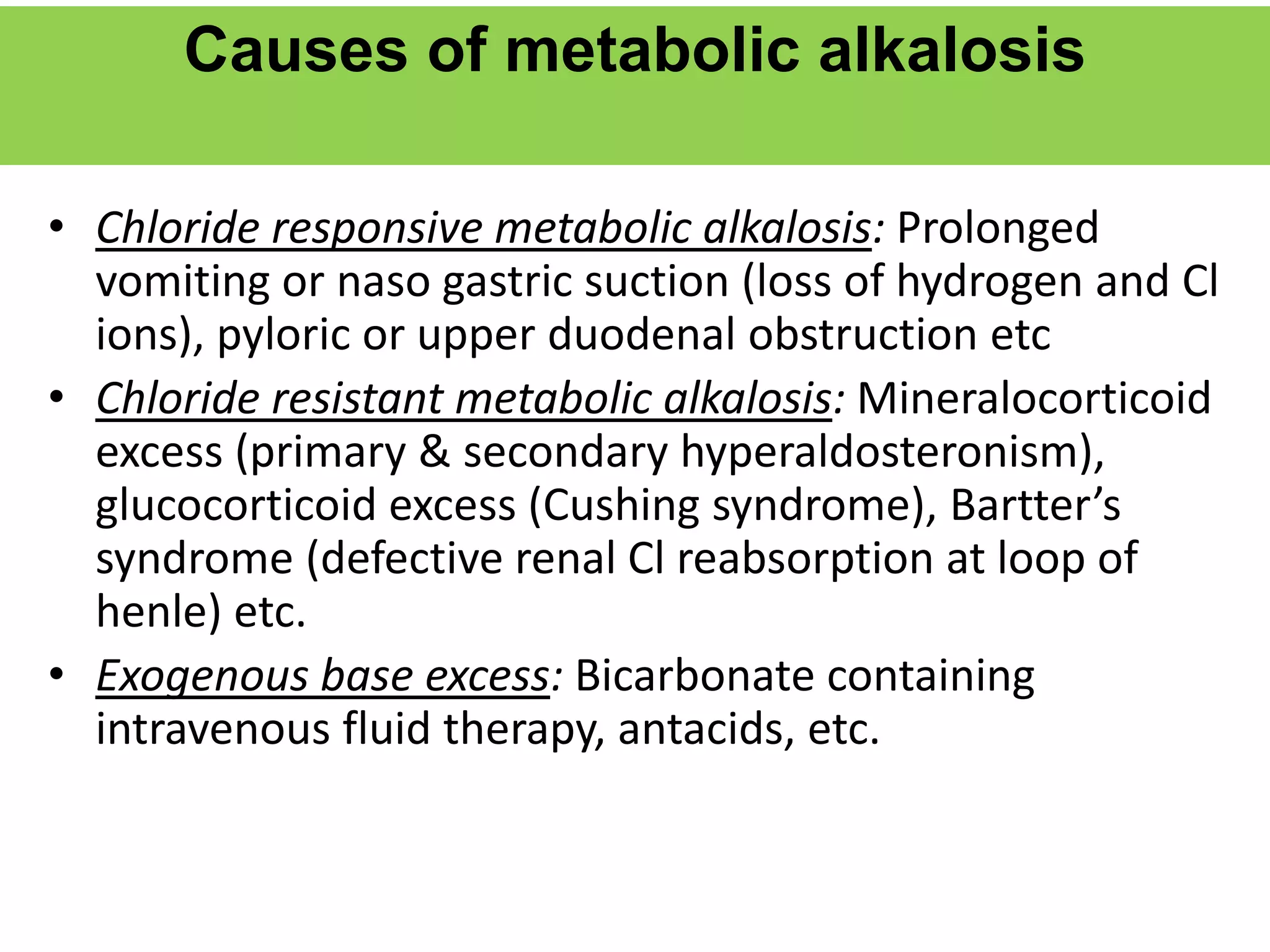
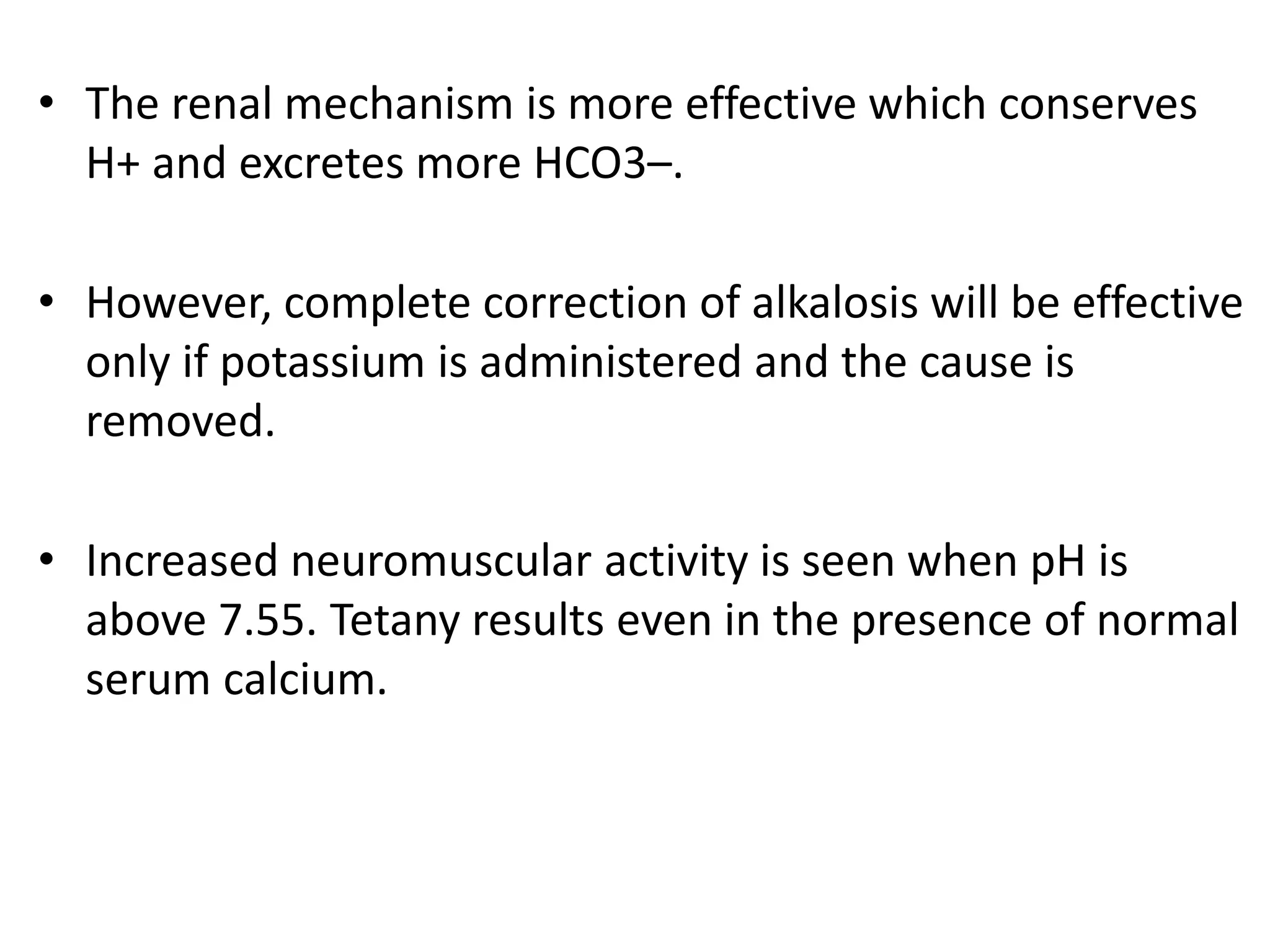
![Metabolic alkalosis
• Characterized by a primary increase in serum [HCO3
-].
• Alkalosis occurs when:
Excess base is added
Acid is lost.
• All these will lead to an excess of bicarbonate, so that the
ratio becomes more than 20.](https://image.slidesharecdn.com/acidbasebalancerelateddisorders-copy-220319062558/75/ACID-BASE-BALANCE-AND-RELATED-DISORDERS-Dr-M-PRIYANKA-87-2048.jpg)









![BLOOD GAS ANALYSIS
• Arterial blood gas test measures the dissolved oxygen and
carbon dioxide in the arterial blood
• Reveals the acid-base state and how well the oxygen is
being carried to the body.
• The pH is the measurement of free H+ ion concentration in
circulating blood.
• Blood gas analyzers directly measure pH and PCO2, and the
HCO3
– value is calculated from the Henderson–Hasselbalch
equation:
pH= 6.1 + log [HCO3
-/0.3 x PCO2]](https://image.slidesharecdn.com/acidbasebalancerelateddisorders-copy-220319062558/75/ACID-BASE-BALANCE-AND-RELATED-DISORDERS-Dr-M-PRIYANKA-101-2048.jpg)