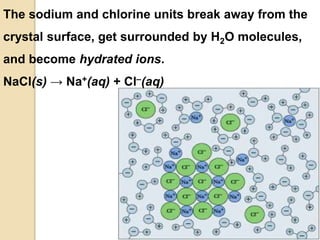
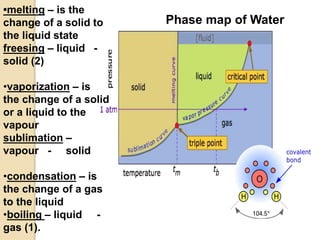
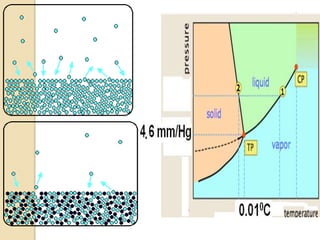
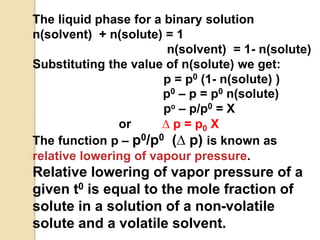
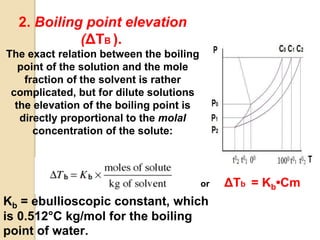
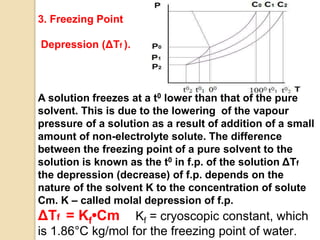
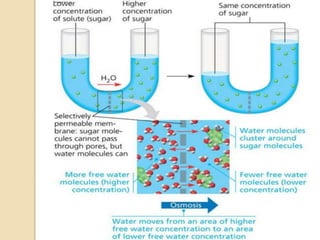
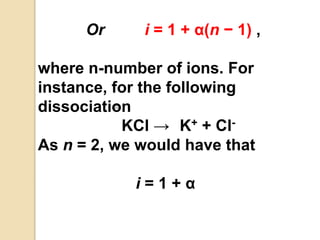
1. A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. Solubility refers to the ability of a solute to dissolve in a solvent.
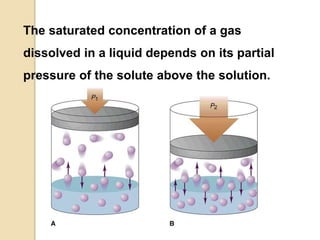
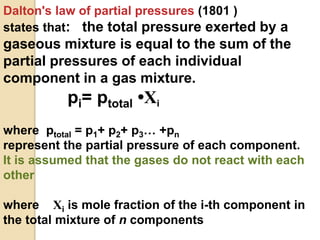
2. Henry's law states that the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of the gas above the liquid at a constant temperature.
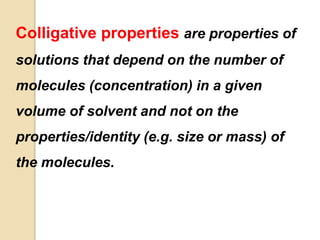
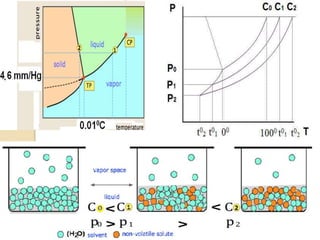
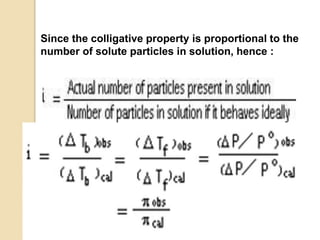
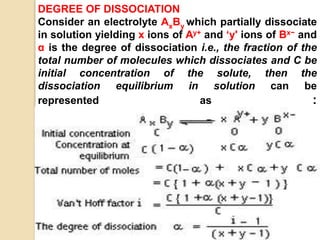
3. Colligative properties depend on the number of solute particles in solution, not their identity, and include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.